Data Table A) Mass of crucible (g) 20.442 B) Mass of magnesium and crucible (g) 20.717 C) Mass of magnesium (g) 0.215g D) Mass of magnesium oxide (g) First weighing: 0.453 Second weighing: 0.455 Average mass (g) 0.454g E) Mass of oxygen (g) 0.179g Calculations Watch this video only if you need help in analyzing the data collected. you https://www.youtube.com/watch?v3DVpgb1Y321WA 1. Atomic mass of magnesium from the Periodic Table is 24.305 g/mol. x = Moles of Mg used in this experiment = 0.0113145 mol of mg (Show your calculation)
Data Table A) Mass of crucible (g) 20.442 B) Mass of magnesium and crucible (g) 20.717 C) Mass of magnesium (g) 0.215g D) Mass of magnesium oxide (g) First weighing: 0.453 Second weighing: 0.455 Average mass (g) 0.454g E) Mass of oxygen (g) 0.179g Calculations Watch this video only if you need help in analyzing the data collected. you https://www.youtube.com/watch?v3DVpgb1Y321WA 1. Atomic mass of magnesium from the Periodic Table is 24.305 g/mol. x = Moles of Mg used in this experiment = 0.0113145 mol of mg (Show your calculation)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 10QRT
Related questions
Question
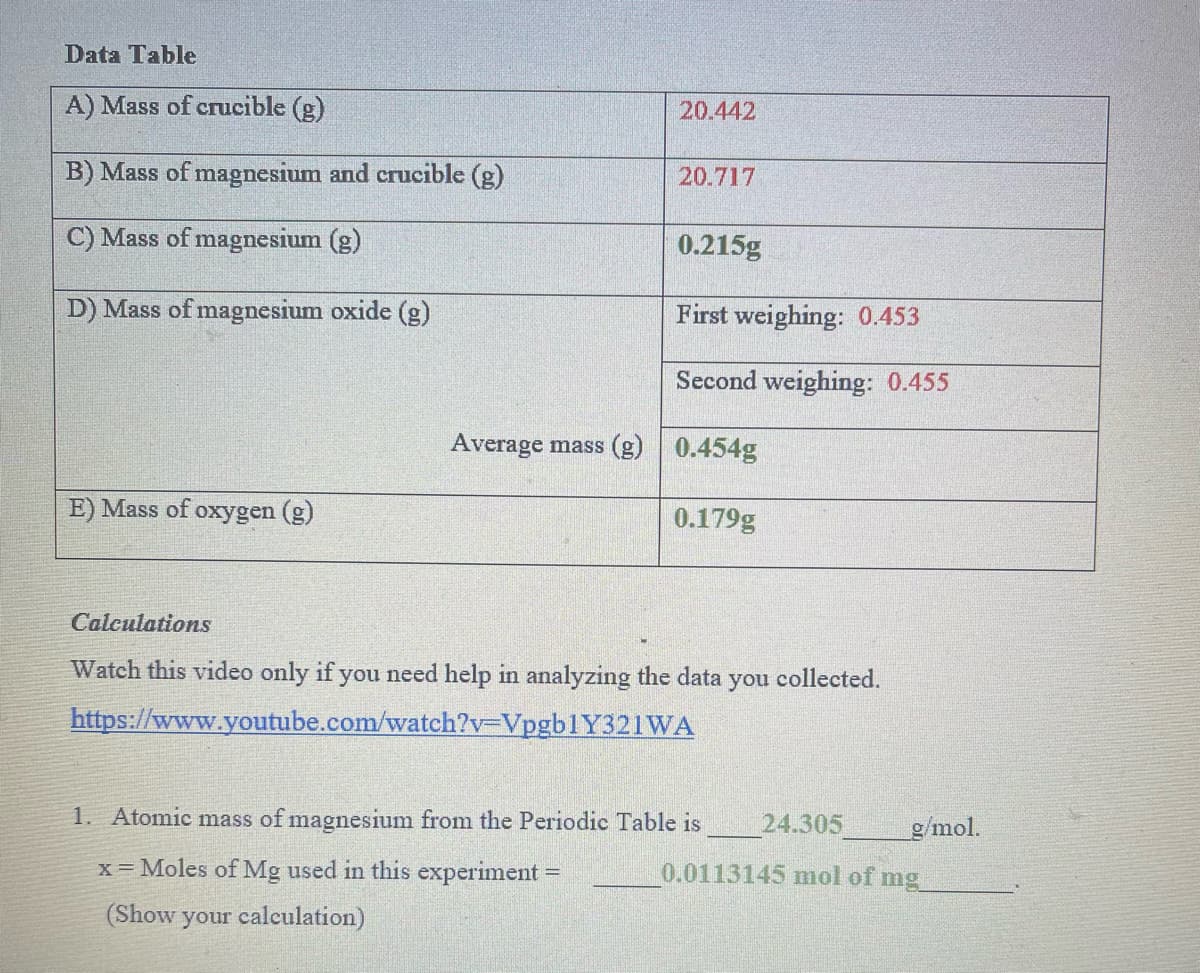
Transcribed Image Text:Data Table
A) Mass of crucible (g)
20.442
B) Mass of magnesium and crucible (g)
20.717
C) Mass of magnesium (g)
0.215g
D) Mass of magnesium oxide (g)
First weighing: 0.453
Second weighing: 0.455
Average mass (g)
0.454g
E) Mass of oxygen (g)
0.179g
Calculations
Watch this video only if you need help in analyzing the data you collected.
https://www.youtube.com/watch?v3DVpgb1Y321WA
1. Atomic mass of magnesium from the Periodic Table is
24.305
g/mol.
x = Moles of Mg used in this experiment =
0.0113145 mol of mg
%3D
(Show your calculation)

Transcribed Image Text:6. Calculate the theoretical %O in magnesium oxide.
At.mass 0 (g/mol)
X 100
Molar mass Mg0 (g/mol)
7. Calculate the experinmental %O in the oxide.
mass 0 (g)
%0 (еxp)
x 100
mass MgO (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning