. Starting from the universal gas constant (R = U.0821 , determine ue volume (In L) occupled by 2.45 moles of an ideal gas at 32.0°C and a pressure of 0.956 atm. mol-K
. Starting from the universal gas constant (R = U.0821 , determine ue volume (In L) occupled by 2.45 moles of an ideal gas at 32.0°C and a pressure of 0.956 atm. mol-K
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
Transcribed Image Text:1 Drill-1-Set-B.pdf - Google Drive
140
om file/d/168319xWk3VB4pwRANaFP
44 RA w
O CHEM 18.1 - 29L KAS 1- W1
O MCB 11 Laboratory
BS ABT FILES
AGRI 41 WSC Lectu..
9 SAIS
A Open with Adobe Acrobat DC
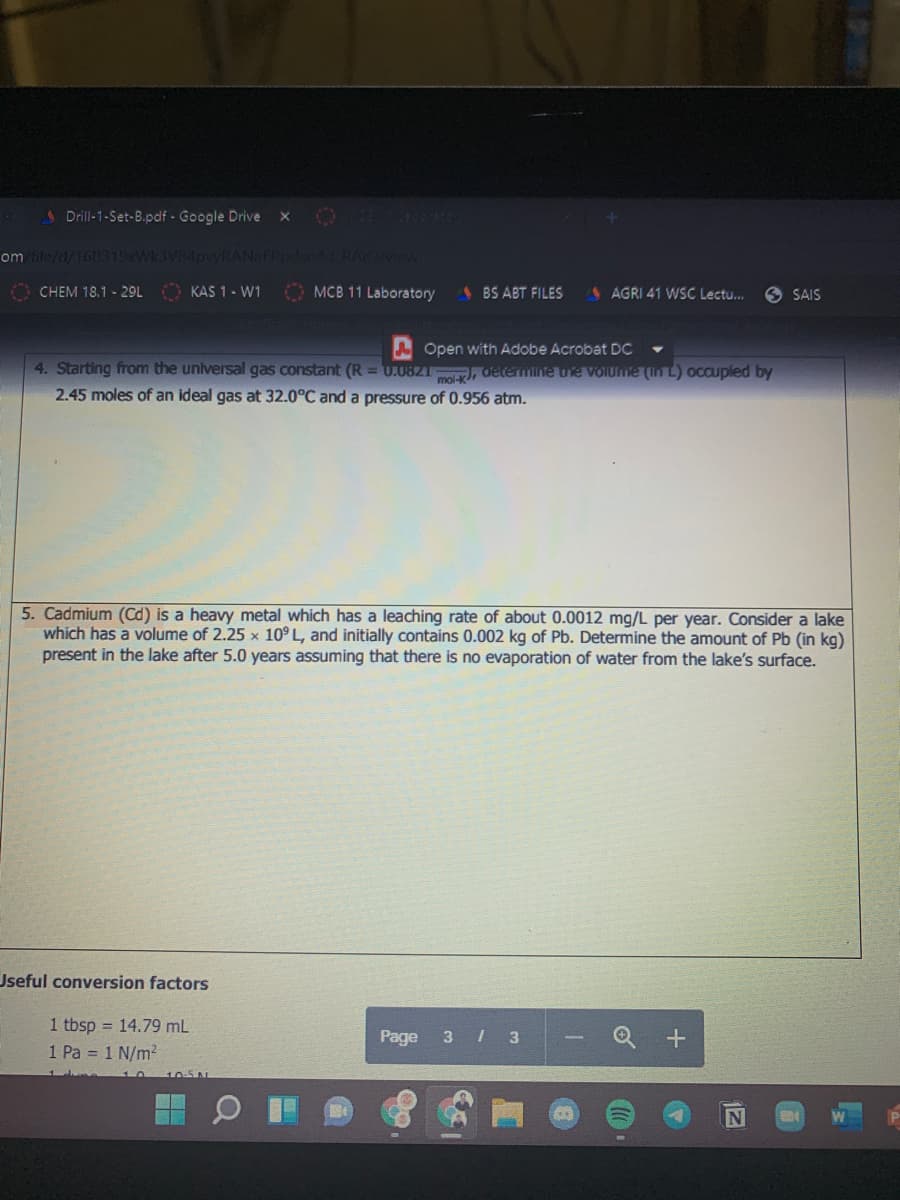
4. Starting from the universal gas constant (R = U.0821 , determine ue Volume (In L) occupled by
2.45 moles of an ideal gas at 32.0°C and a pressure of 0.956 atm.
5. Cadmium (Cd) is a heavy metal which has a leaching rate of about 0.0012 mg/L per year. Consider a lake
which has a volume of 2.25 x 10° L, and initially contains 0.002 kg of Pb. Determine the amount of Pb (in kg)
present in the lake after 5.0 years assuming that there is no evaporation of water from the lake's surface.
Jseful conversion factors
1 tbsp = 14.79 mL
1 Pa = 1 N/m²
Page
3 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning