Data Table for the Laboratory Activity Mass of Mass of Mixture KNO3 H₂O Temperature of Recrystallization (°C) (g) (g) 1 4.0 10.0 24 2 5.0 32 10.0 45 3 7.5 10.0 4 10.0 10.0 58 Refer to Figure 1 and answer the following Question: Compare the freezing point of mixture 4 at 1.0 atm to the freezing point of water at 1.0 atm. Answer
Data Table for the Laboratory Activity Mass of Mass of Mixture KNO3 H₂O Temperature of Recrystallization (°C) (g) (g) 1 4.0 10.0 24 2 5.0 32 10.0 45 3 7.5 10.0 4 10.0 10.0 58 Refer to Figure 1 and answer the following Question: Compare the freezing point of mixture 4 at 1.0 atm to the freezing point of water at 1.0 atm. Answer
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.96PAE: 9.96 Most first aid "cold packs" are based on the endothermic dissolution of ammonium nitrate in...
Related questions
Question
100%
Transcribed Image Text:2
21ww.xnew bojuti ai nom/lo od 1
To omon
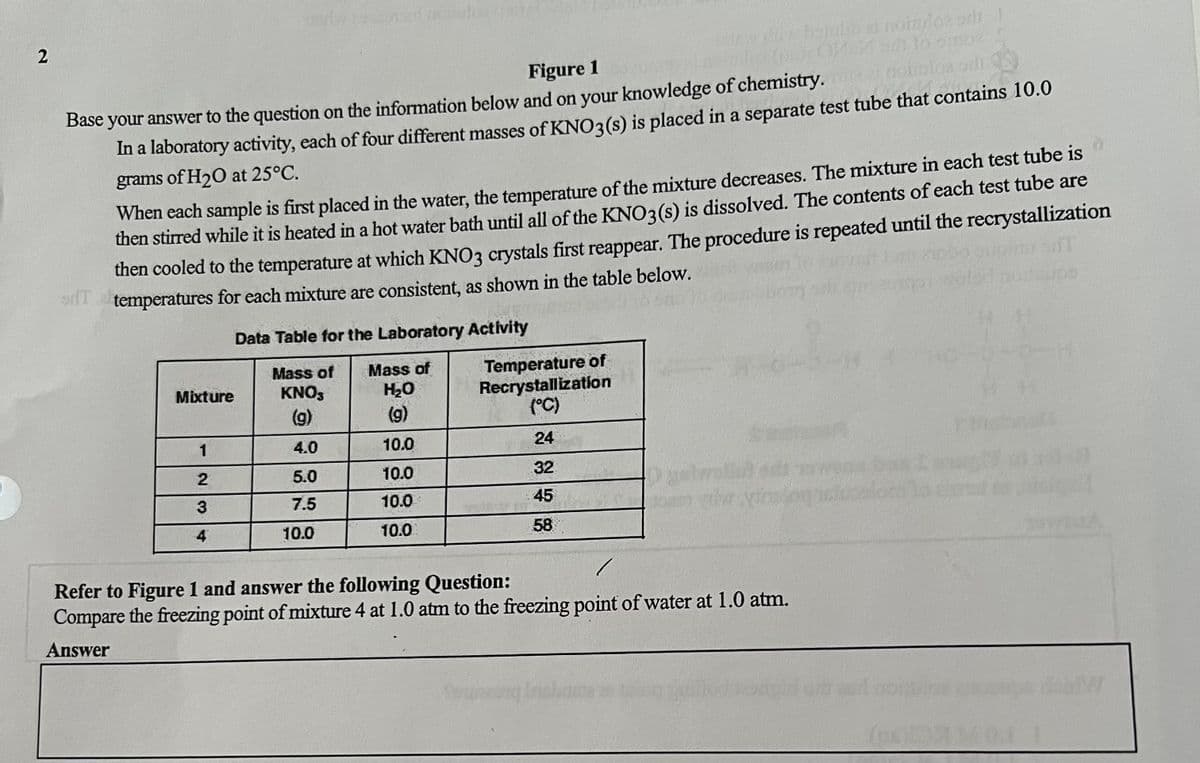
Figure 1
Base your answer to the question on the information below and on your knowledge of chemistry. The
In a laboratory activity, each of four different masses of KNO3(s) is placed in a separate test tube that contains 10.0
grams of H₂O at 25°C.
When each sample is first placed in the water, the temperature of the mixture decreases. The mixture in each test tube is
then stirred while it is heated in a hot water bath until all of the KNO3(s) is dissolved. The contents of each test tube are
then cooled to the temperature at which KNO3 crystals first reappear. The procedure is repeated until the recrystallization
temperatures for each mixture are consistent, as shown in the table below.
Data Table for the Laboratory Activity
Mass of Mass of
Temperature of
Recrystallization
Mixture
KNO3
H₂O
(g)
(9)
(°C)
1
4.0
10.0
24
5.0
10.0
32
twoll
wana ban
7.5
10.0
45
4
10.0
10.0
58
Refer to Figure 1 and answer the following Question:
Compare the freezing point of mixture 4 at 1.0 atm to the freezing point of water at 1.0 atm.
Answer
PURE
23
(DE) 10.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning