Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.47QAP
Related questions
Question
In lab a student calculates the following equilibrium concentrations for Fe ( aq ) 3 + + SCN ( aq ) − ⇋ FeSCN ( aq ) 2 + at each of the specified temperatures. Calculate Δ H ∘ in units of kJ mol. Report your answer to two places after the decimal.
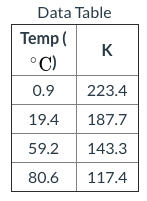
Transcribed Image Text:Data Table
Temp (
K
°C)
0.9
223.4
19.4
187.7
59.2
143.3
80.6
117.4
Expert Solution
Step 1
The Van’t Hoff equation is given as :
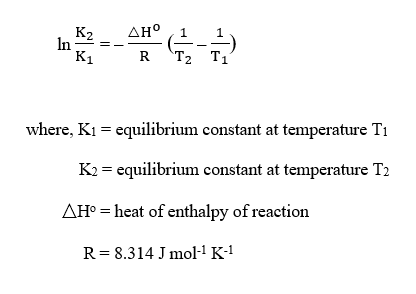
Step 2
From the given data table,
T1 = 0.9 oC = (273 + 0.9) K = 273.9 K
K1 = 223.4
T2 = 19.4 oC = (273 + 19.4) K = 292.4 K
K2 = 187.7
△Ho = ?
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning