Using the law of mass action, write the equilibrium expression for each of the following reactions. (a) 8 H2(g) + Sg(s) 2 8 H,S(g) (b) C(s) + H,O(€) + Cl2(g) = COCl,(g) + H,(g) (c) CaCO3(s) 2 CaO(s) + CO2(g) (d) 3 C,H2(g) = CH(t) Acti
Using the law of mass action, write the equilibrium expression for each of the following reactions. (a) 8 H2(g) + Sg(s) 2 8 H,S(g) (b) C(s) + H,O(€) + Cl2(g) = COCl,(g) + H,(g) (c) CaCO3(s) 2 CaO(s) + CO2(g) (d) 3 C,H2(g) = CH(t) Acti
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.31E
Related questions
Question
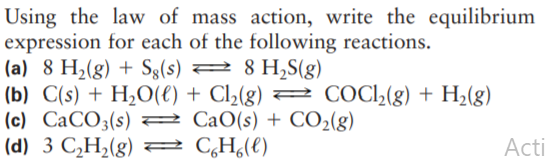
Transcribed Image Text:Using the law of mass action, write the equilibrium
expression for each of the following reactions.
(a) 8 H2(g) + Sg(s) 2 8 H,S(g)
(b) C(s) + H,O(€) + Cl2(g) = COCl,(g) + H,(g)
(c) CaCO3(s) 2 CaO(s) + CO2(g)
(d) 3 C,H2(g) = CH(t)
Acti
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax