Date: Name: BaCl2 BaCl2*H₂O BaCl2*2H₂O Sum atomic 208.23 208.23 208.23 mass of barium chloride (g) Sum of atomic mass of water 0 18.02 x 1 = 18.02 (g) Sum of atomic mass of hydrate (g) 208.23 (208.23 +18.02) = 226.25 C = C - 2006. 09.06 - 2005 Percent water 0% (18.02/226.25) x in the hydrate 100 = (%) 7.96 % Per. BaCl2*3H₂O 208.23
Date: Name: BaCl2 BaCl2*H₂O BaCl2*2H₂O Sum atomic 208.23 208.23 208.23 mass of barium chloride (g) Sum of atomic mass of water 0 18.02 x 1 = 18.02 (g) Sum of atomic mass of hydrate (g) 208.23 (208.23 +18.02) = 226.25 C = C - 2006. 09.06 - 2005 Percent water 0% (18.02/226.25) x in the hydrate 100 = (%) 7.96 % Per. BaCl2*3H₂O 208.23
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.22PAE
Related questions
Question
100%
a) Calculate the theoretical percent water for each value of n--divide the sum of the atomic masses due to the water molecules by the sum of all the atomic masses in the hydrate, and multiply the result by 100. Complete the table.
b) Compare the percent water in the hydrate with the theoretical values calculated for different values of n. Complete the table
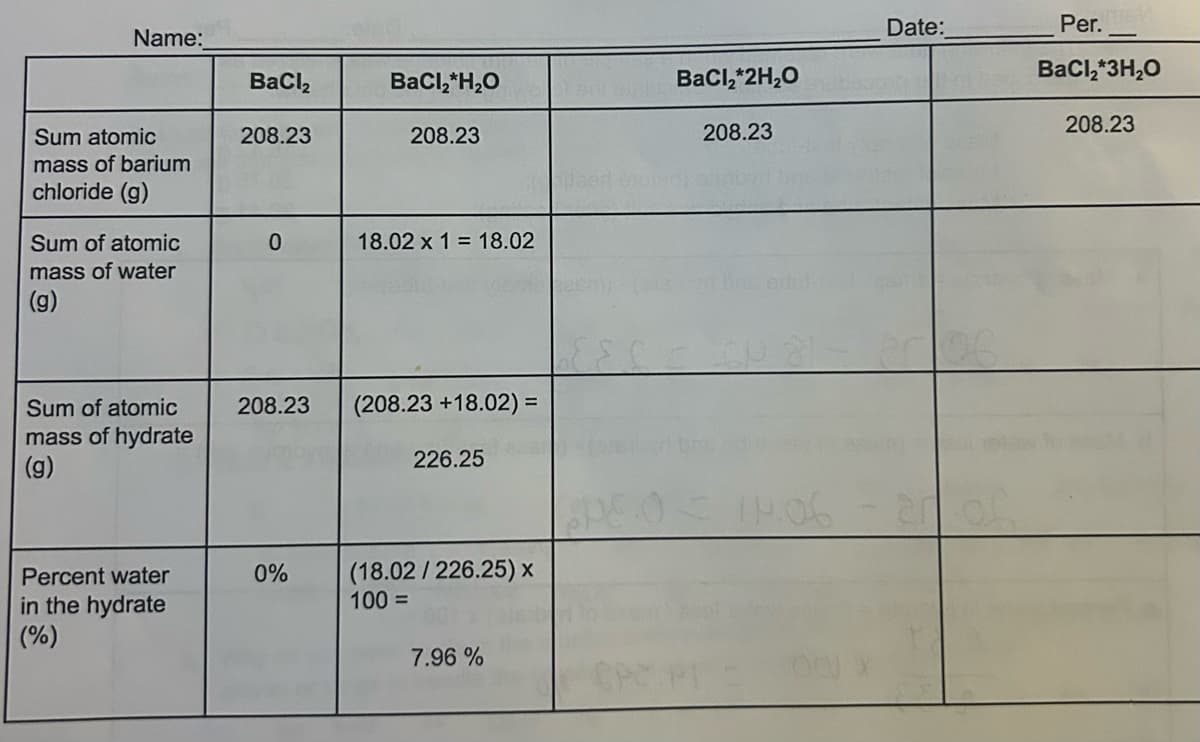
Transcribed Image Text:Date:
Name:
BaCl2
BaCl2*H₂O
BaCl2*2H₂O
Sum atomic
208.23
208.23
208.23
mass of barium
chloride (g)
Sum of atomic
mass of water
0
18.02 x 1 = 18.02
(g)
Sum of atomic
mass of hydrate
(g)
208.23
(208.23 +18.02) =
226.25
C = C - 2006.
09.06 - 2005
Percent water
0%
(18.02/226.25) x
in the hydrate
100 =
(%)
7.96 %
Per.
BaCl2*3H₂O
208.23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning