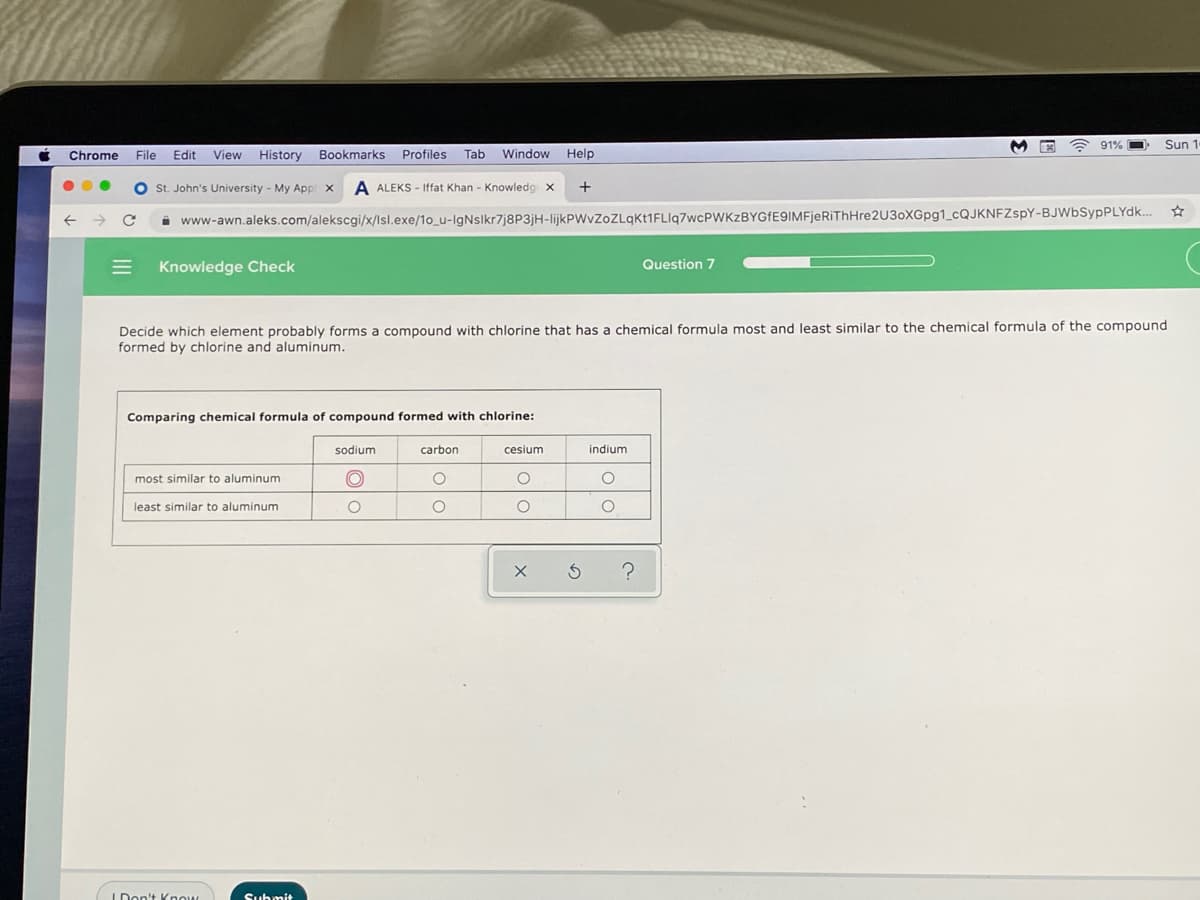
Decide which element probably forms a compound with chlorine that has a chemical formula most and least similar to the chemical formula of the compound formed by chlorine and aluminum. Comparing chemical formula of compound formed with chlorine: sodium carbon cesium indium most similar to aluminum least similar to aluminum
Decide which element probably forms a compound with chlorine that has a chemical formula most and least similar to the chemical formula of the compound formed by chlorine and aluminum. Comparing chemical formula of compound formed with chlorine: sodium carbon cesium indium most similar to aluminum least similar to aluminum
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 134CP
Related questions
Question
Transcribed Image Text:91%
Sun
Chrome
File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
O st. John's University - My Appl x
A ALEKS - Iffat Khan - Knowledg
+
i www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNsIkr7j8P3jH-lijkPWvZOZLqkt1FLIq7wcPWKzBYGfE9IMFjeRiThHre2U3oXGpg1_cQJKNFZspY-BJWbSypPLYdk.. *
Knowledge Check
Question 7
Decide which element probably forms a compound with chlorine that has a chemical formula most and least similar to the chemical formula of the compound
formed by chlorine and aluminum.
Comparing chemical formula of compound formed with chlorine:
sodium
carbon
cesium
indium
most similar to aluminum
least similar to aluminum
IDon't Know
Suhmit
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning