Density of Water Temperature of Water 25 °C Density of water (from table in laboratory materia.s) 0.9970 g/mL TRIAL 1 TRIAL 2 TRIAL 3 Mass of empty beaker 52.81 g 52.81 g 52.81 g (measured) Mass of beaker and water 62.79 g 62.81 g 62.78 g (measured) Mass of water (measured) Volume of water 10.00 mL 10.00 mL 10.00 mL (measured) Density of water (calculated) CALCULATIONS: Show all your work and use correct number of significant figures.
Density of Water Temperature of Water 25 °C Density of water (from table in laboratory materia.s) 0.9970 g/mL TRIAL 1 TRIAL 2 TRIAL 3 Mass of empty beaker 52.81 g 52.81 g 52.81 g (measured) Mass of beaker and water 62.79 g 62.81 g 62.78 g (measured) Mass of water (measured) Volume of water 10.00 mL 10.00 mL 10.00 mL (measured) Density of water (calculated) CALCULATIONS: Show all your work and use correct number of significant figures.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 109AP: For a material to float on the surface of water, the material must have a density less than that of...
Related questions
Question
100%
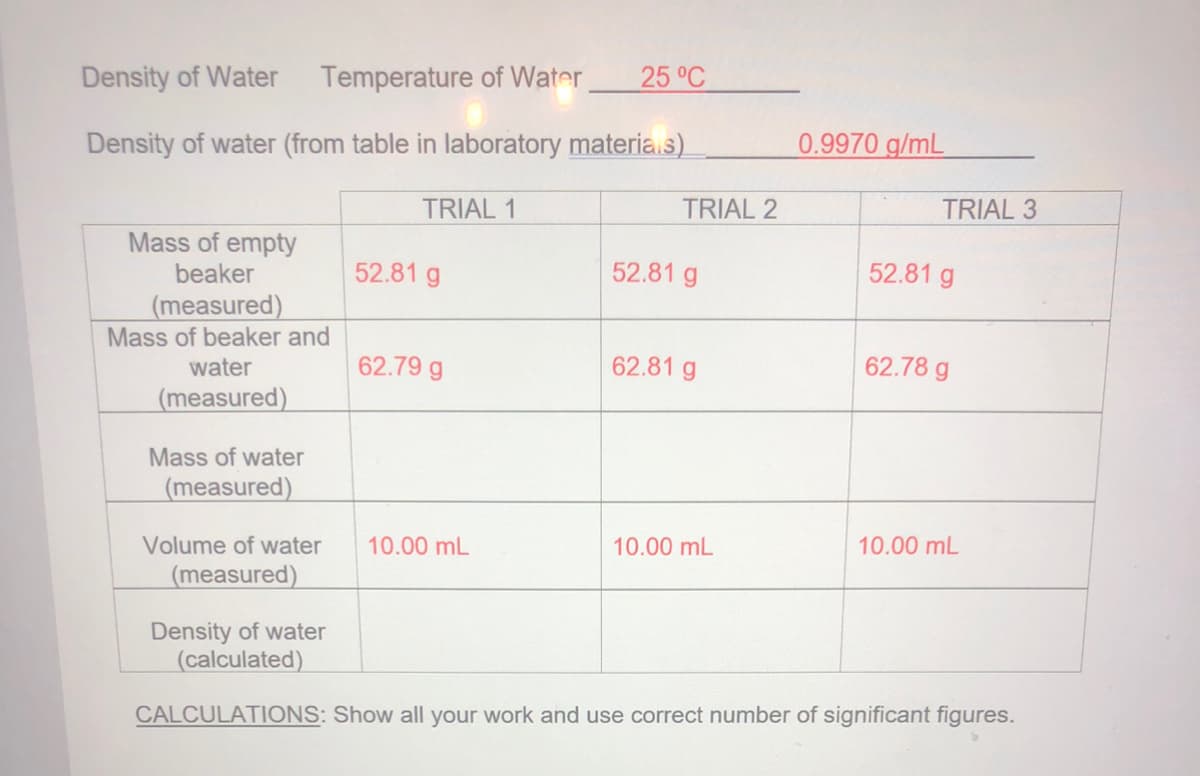
Transcribed Image Text:Density of Water
Temperature of Water
25 °C
Density of water (from table in laboratory materia.s)
0.9970 g/mL
TRIAL 1
TRIAL 2
TRIAL 3
Mass of empty
beaker
52.81 g
52.81 g
52.81 g
(measured)
Mass of beaker and
water
62.79 g
62.81 g
62.78 g
(measured)
Mass of water
(measured)
Volume of water
10.00 mL
10.00 mL
10.00 mL
(measured)
Density of water
(calculated)
CALCULATIONS: Show all your work and use correct number of significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning