Derive the seven general property equation of the following thermodynamics processes: a. ISOMETRIC PROCESS - Any Process relation - Work Non-flow - Internal Energy - Heat Transferred - Enthalphy - Entropy - Work Steady flow refer with the picture. Show the detailed explanation please
Derive the seven general property equation of the following thermodynamics processes: a. ISOMETRIC PROCESS - Any Process relation - Work Non-flow - Internal Energy - Heat Transferred - Enthalphy - Entropy - Work Steady flow refer with the picture. Show the detailed explanation please
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter6: Forced Convection Over Exterior Surfaces
Section: Chapter Questions
Problem 6.25P
Related questions
Question
Derive the seven general property equation of the following thermodynamics processes:
a. ISOMETRIC PROCESS
- Any Process relation
- Work Non-flow
- Internal Energy
- Heat Transferred
- Enthalphy
- Entropy
- Work Steady flow
refer with the picture. Show the detailed explanation please
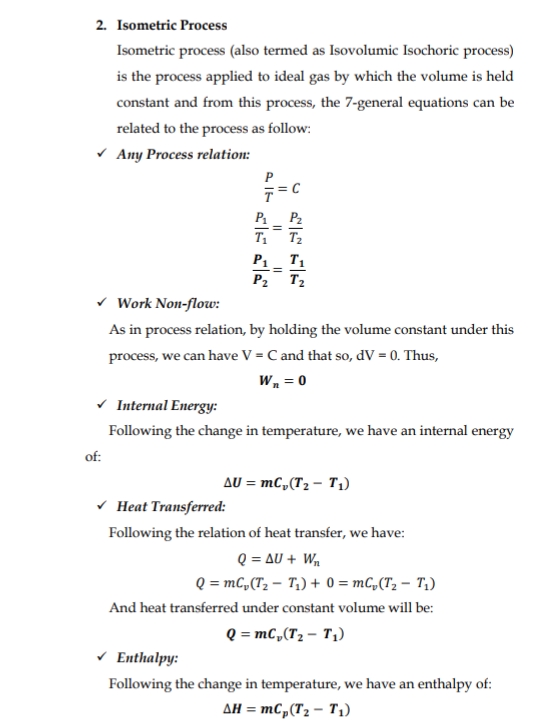
Transcribed Image Text:2. Isometric Process
Isometric process (also termed as Isovolumic Isochoric process)
is the process applied to ideal gas by which the volume is held
constant and from this process, the 7-general equations can be
related to the process as follow:
v Any Process relation:
P
P2
T
P1 _ T1
P2 T2
v Work Non-flow:
As in process relation, by holding the volume constant under this
process, we can have V = C and that so, dV = 0. Thus,
W, = 0
v Internal Energy:
Following the change in temperature, we have an internal energy
of:
AU = mC„(T2 – T;)
• Heat Transferred:
Following the relation of heat transfer, we have:
Q = AU + Wn
Q = mC,(T, – T,) + 0 = mC,(T; – T,)
And heat transferred under constant volume will be:
Q = mC,(T2 – T1)
v Enthalpy:
Following the change in temperature, we have an enthalpy of:
AH = mC,(T2 – T1)

Transcribed Image Text:v Entropy:
Following the guiding equation as represented by the integral
above
we will have:
² dQ
S =
wherein we have:
dQ = mCvdT
r² dT
S = mCv
Evaluating the integral, we will obtain:
AS =
- morm) - mevm)
mCvln|
v Work Steady flow:
For work steady flow, we follow the integral helow:
W, = - [var
VdP
Also, from process definition:
PV" = C where n = ∞ (isometric, isochoric)
V = C
Since the volume is constant, we can rewrite the integral above in
the
form,
W,
dP
Evaluating, we will have:
W, = -C(P2 – P,)
Substituting V = C,
W, = -V(P2 – P1) = V(P1 – P2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning