The Antoine Equation can be used to estimate vapor pressure at various temperatures for different substances using sets of empirically-derived constants. The equation can be written as: Where for water A = 8.07131, B = 1730.63, C = 233.426, T = temperature [°C], and P = vapor pressure [mmHg]. Note that with these constants the temperature and pressure must be provided in °C and mmHg, respectively. Using the Antoine Equation and the provided constants, what atmospheric pressure (provided in terms of % of standard atmospheric pressure) will permit water to boil at 75 °C?
The Antoine Equation can be used to estimate vapor pressure at various temperatures for different substances using sets of empirically-derived constants. The equation can be written as: Where for water A = 8.07131, B = 1730.63, C = 233.426, T = temperature [°C], and P = vapor pressure [mmHg]. Note that with these constants the temperature and pressure must be provided in °C and mmHg, respectively. Using the Antoine Equation and the provided constants, what atmospheric pressure (provided in terms of % of standard atmospheric pressure) will permit water to boil at 75 °C?
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter29: Troubleshooting And Typical Operating Conditions For Commercial refrigeration
Section: Chapter Questions
Problem 16RQ
Related questions
Question
The Antoine Equation can be used to estimate vapor pressure at various temperatures for different substances using sets of empirically-derived constants. The equation can be written as:
Where for water A = 8.07131, B = 1730.63, C = 233.426, T = temperature [°C], and P = vapor pressure [mmHg]. Note that with these constants the temperature and pressure must be provided in °C and mmHg, respectively.
Using the Antoine Equation and the provided constants, what atmospheric pressure (provided in terms of % of standard atmospheric pressure) will permit water to boil at 75 °C?
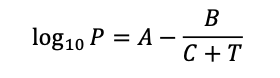
Transcribed Image Text:B
log10 P = A -
C + T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning