Design a buffer that has a pH of 6.00 using one of the weak base/conjugate acid systems shown below. Weak Base Conjugate Acid CH3NH2 4.2x10-4 CH3NH3+ 2.4x10-11 10.62 C6H1503N 5.9x10-7 C6H1503NH+ 1.7×10-8 7.77 C5H5N 1.5x10-9 C₂H5NH+ 6.7x10-6 5.17 Kb Ka pka How many grams of the bromide salt of the conjugate acid must be combined with how many grams of the weak base, to produce 1.00 L of a buffer that is 1.00 M in the weak base? grams bromide salt of conjugate acid = grams weak base =
Design a buffer that has a pH of 6.00 using one of the weak base/conjugate acid systems shown below. Weak Base Conjugate Acid CH3NH2 4.2x10-4 CH3NH3+ 2.4x10-11 10.62 C6H1503N 5.9x10-7 C6H1503NH+ 1.7×10-8 7.77 C5H5N 1.5x10-9 C₂H5NH+ 6.7x10-6 5.17 Kb Ka pka How many grams of the bromide salt of the conjugate acid must be combined with how many grams of the weak base, to produce 1.00 L of a buffer that is 1.00 M in the weak base? grams bromide salt of conjugate acid = grams weak base =
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 4RQ: A good buffer generally contains relatively equal concentrations of weak acid and conjugate base. If...
Related questions
Question
Please make sure to double check that it solved for grams bromide salt of conjugate acid, and weak base. I have already wasted a question because they answered it for the base not acid.
Thank you
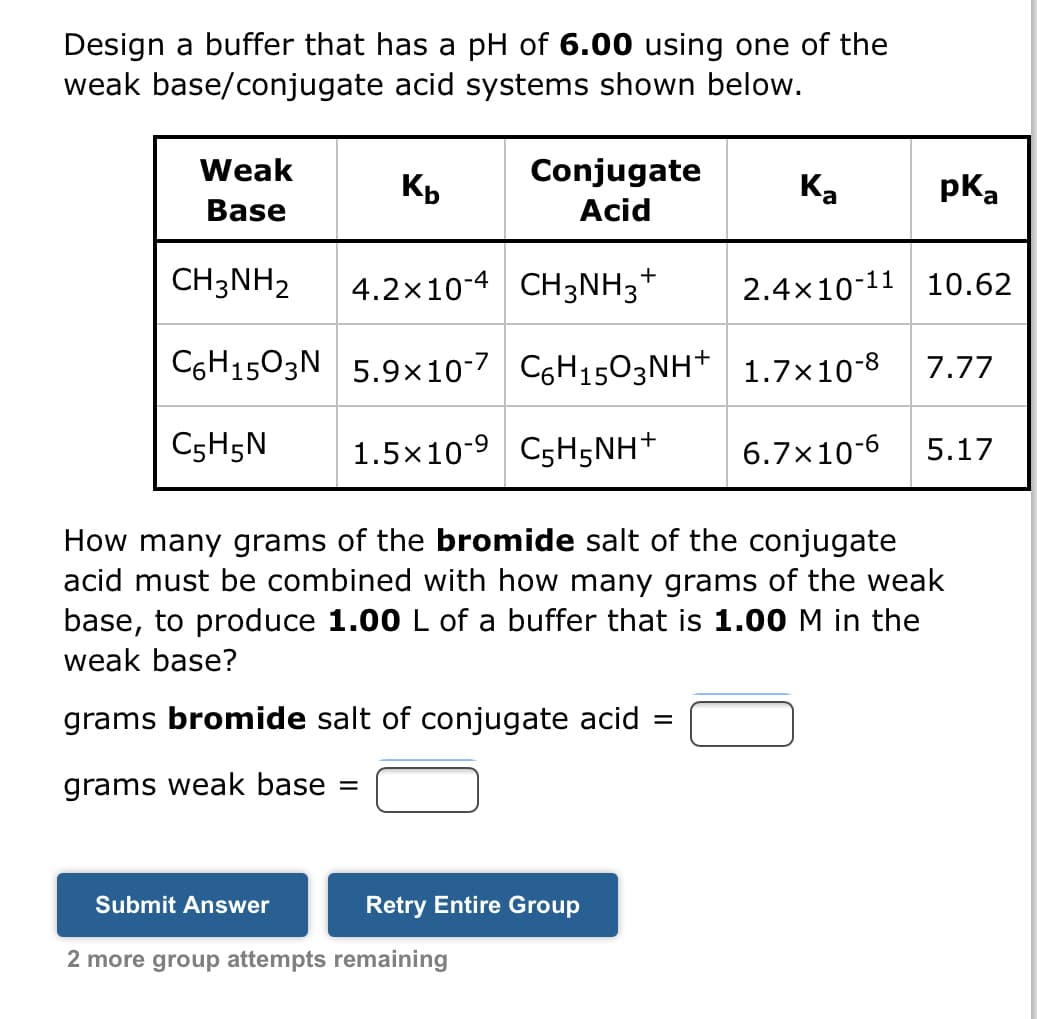
Transcribed Image Text:Design a buffer that has a pH of 6.00 using one of the
weak base/conjugate
acid systems shown below.
Weak
Base
Kb
pka
CH3NH₂
4.2×10-4 CH3NH3† 2.4x10-11 10.62
C6H1503N 5.9x10-7 C6H1503NH+ 1.7×10-8 7.77
C5H5N 1.5x10-9 C5H5NH+ 6.7x10-6 5.17
Submit Answer
Conjugate
Acid
How many grams of the bromide salt of the conjugate
acid must be combined with how many grams of the weak
base, to produce 1.00 L of a buffer that is 1.00 M in the
weak base?
grams bromide salt of conjugate acid =
grams weak base =
Retry Entire Group
2 more group attempts remaining
Ka
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning