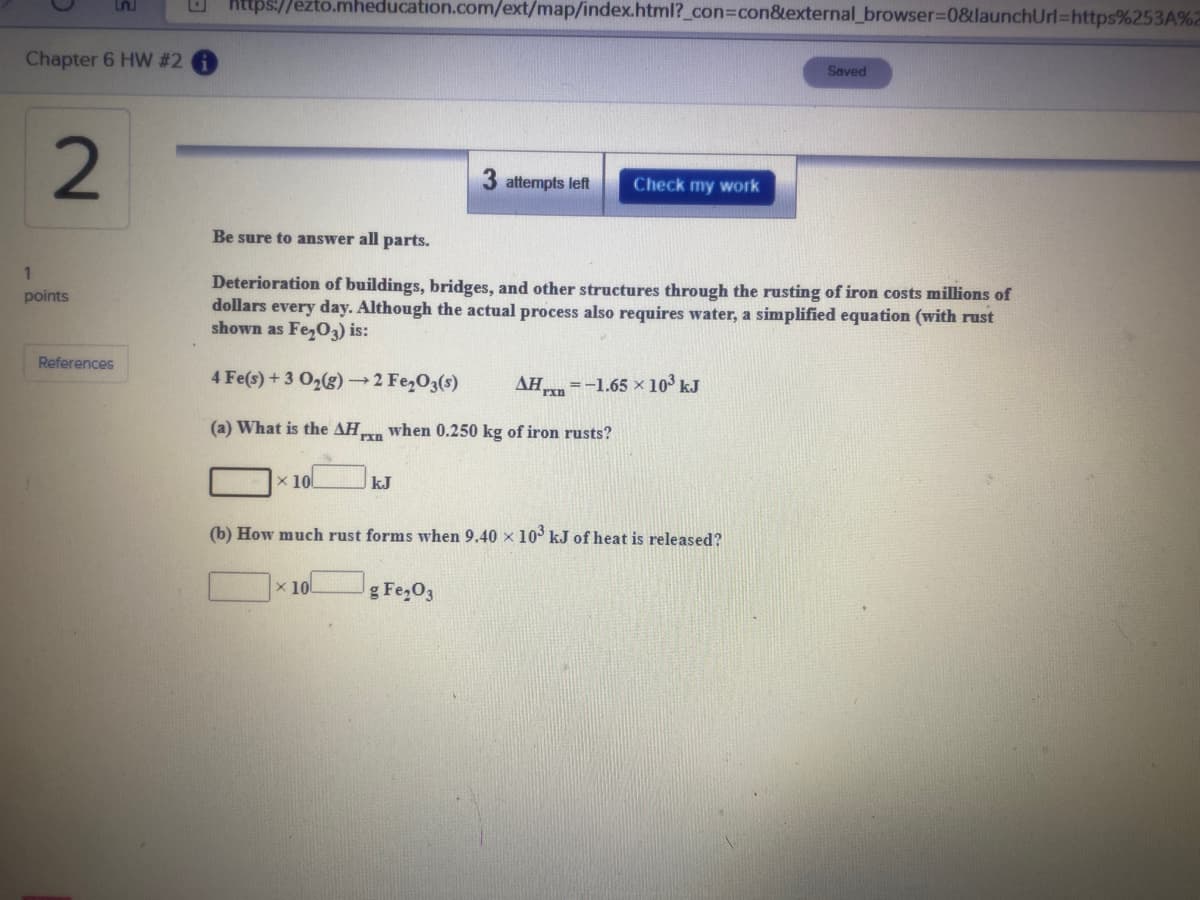
Deterioration of buildings, bridges, and other structures through the rusting of iron costs millions of dollars every day. Although the actual process also requires water, a simplified equation (with rust shown as Fe,O3) is: 4 Fe(s) + 3 02(g) → 2 Fe,O3(s) AH =-1.65 × 10³ kJ (a) What is the AHxn when 0.250 kg of iron rusts? x 10 kJ (b) How much rust forms when 9.40 x 10 kJ of heat is released? x 10 g Fe,O3
Deterioration of buildings, bridges, and other structures through the rusting of iron costs millions of dollars every day. Although the actual process also requires water, a simplified equation (with rust shown as Fe,O3) is: 4 Fe(s) + 3 02(g) → 2 Fe,O3(s) AH =-1.65 × 10³ kJ (a) What is the AHxn when 0.250 kg of iron rusts? x 10 kJ (b) How much rust forms when 9.40 x 10 kJ of heat is released? x 10 g Fe,O3
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.104QE
Related questions
Question
Transcribed Image Text:https://ezto.mheducation.com/ext/map/index.html?_con%3Dcon&external_browser3D0&launchUrl%=Dhttps%253A%2
Chapter 6 HW #2
Saved
2
attempts left
Check my work
Be sure to answer all parts.
1
Deterioration of buildings, bridges, and other structures through the rusting of iron costs millions of
dollars every day. Although the actual process also requires water, a simplified equation (with rust
shown as Fe,O3) is:
points
References
4 Fe(s) + 3 O2(g)→2 Fe,O3(s)
AH =-1.65 × 103 kJ
(a) What is the AH when 0.250 kg of iron rusts?
x 10
kJ
(b) How much rust forms when 9.40 x 10° kJ of heat is released?
x 10
g Fe,O3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning