determination of formic acid by neutralization volumetry, the pH was calculated as a function of the addition of NaOH for the titration of 25.00 mL of 0.1280 mol/L formic acid with NaOH standardized with concentration of 0.0670 mol/L. With reference to this titration curve, determine the pH of the solution after the addition of 5.00 mL of titrant and at the equivalence point. Data: Ka=1.70x10-4. Present the results with two places after the comma and show your calculations. c) Based on the table below, indicate which indicators could be used to determine the end point of this titration.
determination of formic acid by neutralization volumetry, the pH was calculated as a function of the addition of NaOH for the titration of 25.00 mL of 0.1280 mol/L formic acid with NaOH standardized with concentration of 0.0670 mol/L. With reference to this titration curve, determine the pH of the solution after the addition of 5.00 mL of titrant and at the equivalence point. Data: Ka=1.70x10-4. Present the results with two places after the comma and show your calculations. c) Based on the table below, indicate which indicators could be used to determine the end point of this titration.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.34QAP
Related questions
Question
b) In the development of a method for the determination of formic acid by neutralization volumetry, the pH was calculated as a function of the addition of NaOH for the titration of 25.00 mL of 0.1280 mol/L formic acid with NaOH standardized with concentration of 0.0670 mol/L. With reference to this titration curve, determine the pH of the solution after the addition of 5.00 mL of titrant and at the equivalence point. Data: Ka=1.70x10-4. Present the results with two places after the comma and show your calculations.
c) Based on the table below, indicate which indicators could be used to determine the end point of this titration.
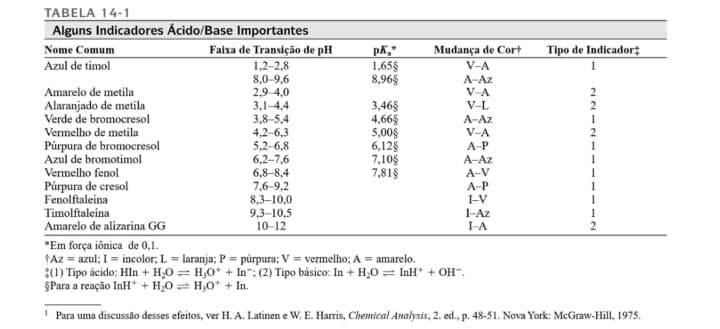
Transcribed Image Text:TABELA 14-1
Alguns Indicadores Ácido/Base Importantes
Nome Comum
Azul de timol
Faixa de Transição de pH
Mudança de Cort
Tipo de Indicador;
1,2-2,8
1,65§
8,96$
V-A
8,0-9,6
2,9 4,0
A-Az
Amarelo de metila
Alaranjado de metila
Verde de bromocresol
V-A
3,1-4,4
3,8-5,4
4,2-6,3
5,2-6,8
6,2-7,6
3,465
4,66$
5,00$
6,125
7,10$
7,81$
V-L
A-Az
V-A
A-P
Vermelho de metila
Púrpura de bromocresol
Azul de bromotimol
A-Az
A-V
Vermelho fenol
6,8-8,4
7,6-9,2
8,3–10,0
9,3-10,5
10-12
Púrpura de cresol
Fenolftaleina
A-P
-V
I-Az
I-A
Timolftaleina
Amarelo de alizarina GG
*Em força iõnica de 0,1.
1Az = azul; I = incolor; L = laranja; P = púrpura; V = vermelho; A = amarelo.
(1) Tipo ácido: HIn + H,0 = H,0* + In-: (2) Tipo básico: In + H,0 = InH* + OH-.
SPara a reação InH* + H,0 = H,0* + In.
Para uma discussão desses efeitos, ver H. A. Latinen e W. E. Harris, Chemical Analysis, 2. ed., p. 48-51. Nova York: McGraw-Hill, 1975.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

