Determination of the Surface Tension of a Liquid by the Capillary Rise Method: A group of students enrolled in a Physical Chemistry Laboratory class performed an experiment on the determination of the surface tension of a liquid by the capillary rise method. The data gathered by the group are documented in the tables below. Solve for the unknown data for #s 1-12. Compounds Average Height, dm Density (e/ml) Water 0.16 0.9998 Benzene 0.08 0.7882 Chloroform 0.03 1.4592 Methanol 0.07 0.8707
Determination of the Surface Tension of a Liquid by the Capillary Rise Method: A group of students enrolled in a Physical Chemistry Laboratory class performed an experiment on the determination of the surface tension of a liquid by the capillary rise method. The data gathered by the group are documented in the tables below. Solve for the unknown data for #s 1-12. Compounds Average Height, dm Density (e/ml) Water 0.16 0.9998 Benzene 0.08 0.7882 Chloroform 0.03 1.4592 Methanol 0.07 0.8707
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 20E: The density of liquid NH3 is 0.64 g/mL; the density of gaseous NH3 at STP is 0.0007 g/mL. Explain...
Related questions
Question
100%
kindly answer only for 7 8 and 9
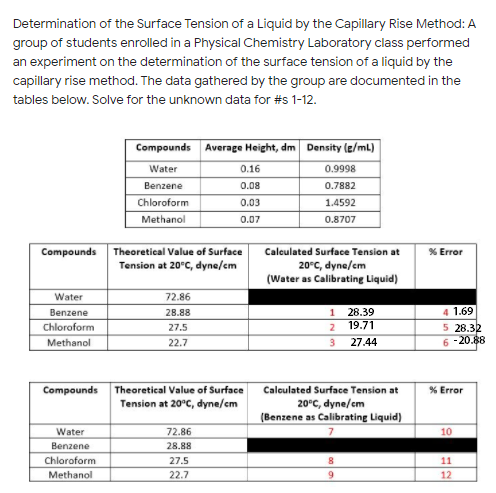
Transcribed Image Text:Determination of the Surface Tension of a Liquid by the Capillary Rise Method: A
group of students enrolled in a Physical Chemistry Laboratory class performed
an experiment on the determination of the surface tension of a liquid by the
capillary rise method. The data gathered by the group are documented in the
tables below. Solve for the unknown data for #s 1-12.
Compounds Average Height, dm Density (e/ml)
Water
0.16
0.9998
Benzene
0.08
0.7882
Chloroform
0.03
1.4592
Methanol
0.07
0.8707
Compounds
Theoretical Value of Surface
Calculated Surface Tension at
% Error
20°C, dyne/cm
(Water as Calibrating Liquid)
Tension at 20°C, dyne/cm
Water
72.86
Benzene
28.88
1
28.39
4 1.69
19.71
5 28.32
6 -20.88
Chloroform
27.5
2
Methanol
22.7
3
27.44
Compounds
Theoretical Value of Surface
Calculated Surface Tension at
% Error
Tension at 20°C, dyne/cm
20°C, dyne/cm
(Benzene as Calibrating Liquid)
Water
72.86
10
Benzene
28.88
Chloroform
27.5
11
Methanol
22.7
9.
12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax