Determine the activation energy, Ea, of the decomposition reaction, CH,CHO → CH, + CO. T (°C) 486 563 k (M-V2 s -1) 0.00105 0.0214 52.7 J/mol a. b. 2040 J/mol 38.4 kJ/mol d. 89.1 kJ/mol 207 kJ/mol e. c.
Determine the activation energy, Ea, of the decomposition reaction, CH,CHO → CH, + CO. T (°C) 486 563 k (M-V2 s -1) 0.00105 0.0214 52.7 J/mol a. b. 2040 J/mol 38.4 kJ/mol d. 89.1 kJ/mol 207 kJ/mol e. c.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter16: Reaction Rates
Section: Chapter Questions
Problem 6STP
Related questions
Question
Practice Pack
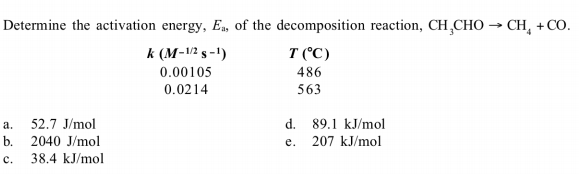
Transcribed Image Text:Determine the activation energy, Ea, of the decomposition reaction, CH,CHO → CH, + CO.
T (°C)
486
563
k (M-V2 s -1)
0.00105
0.0214
52.7 J/mol
a.
b. 2040 J/mol
38.4 kJ/mol
d. 89.1 kJ/mol
207 kJ/mol
e.
c.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Includes step-by-step video
Trending now
This is a popular solution!
Learn your way
Includes step-by-step video
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax