A certain catalyzed reaction is known to have an activation energy E-53.0 kJ/mol. Furthermore, the rate of this reaction is measured at 359. K and found to be 1.9 × 10² M/s. Use this information to answer the questions in the table below. Suppose the concentrations of all reactants is kept the same, but the temperature is lowered by 5% from 359. K to 341. K. The rate will choose one v How will the rate of the reaction change? Suppose the concentrations of all reactants is kept the same, but the catalyst is removed, which has the effect of raising the activation. energy by 5%, from 53.0 kJ/mol to The rate will ✓ choose one 55.7 kJ/mol. How will the rate of the reaction change? stay the same rise about 5% rise more than 5% rise less than 5% fall about 5% fall more than 5% fall less than 5%
A certain catalyzed reaction is known to have an activation energy E-53.0 kJ/mol. Furthermore, the rate of this reaction is measured at 359. K and found to be 1.9 × 10² M/s. Use this information to answer the questions in the table below. Suppose the concentrations of all reactants is kept the same, but the temperature is lowered by 5% from 359. K to 341. K. The rate will choose one v How will the rate of the reaction change? Suppose the concentrations of all reactants is kept the same, but the catalyst is removed, which has the effect of raising the activation. energy by 5%, from 53.0 kJ/mol to The rate will ✓ choose one 55.7 kJ/mol. How will the rate of the reaction change? stay the same rise about 5% rise more than 5% rise less than 5% fall about 5% fall more than 5% fall less than 5%
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.99QE
Related questions
Question
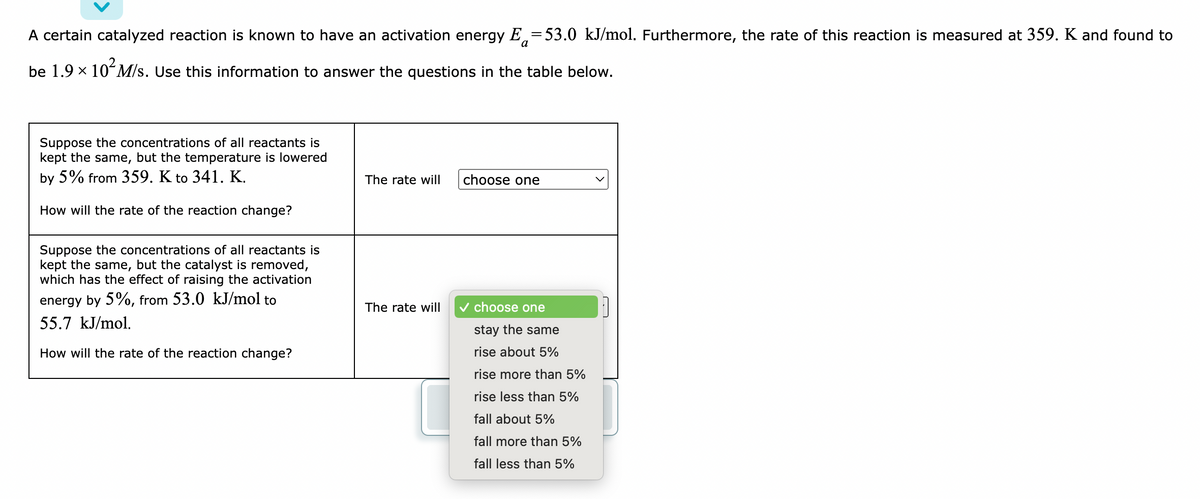
Transcribed Image Text:A certain catalyzed reaction is known to have an activation energy E53.0 kJ/mol. Furthermore, the rate of this reaction is measured at 359. K and found to
be 1.9 × 10² M/s. Use this information to answer the questions in the table below.
Suppose the concentrations of all reactants is
kept the same, but the temperature is lowered
by 5% from 359. K to 341. K.
The rate will
choose one
How will the rate of the reaction change?
Suppose the concentrations of all reactants is
kept the same, but the catalyst is removed,
which has the effect of raising the activation
energy by 5%, from 53.0 kJ/mol to
The rate will
55.7 kJ/mol.
✓ choose one
stay the same
How will the rate of the reaction change?
rise about 5%
rise more than 5%
rise less than 5%
fall about 5%
fall more than 5%
fall less than 5%
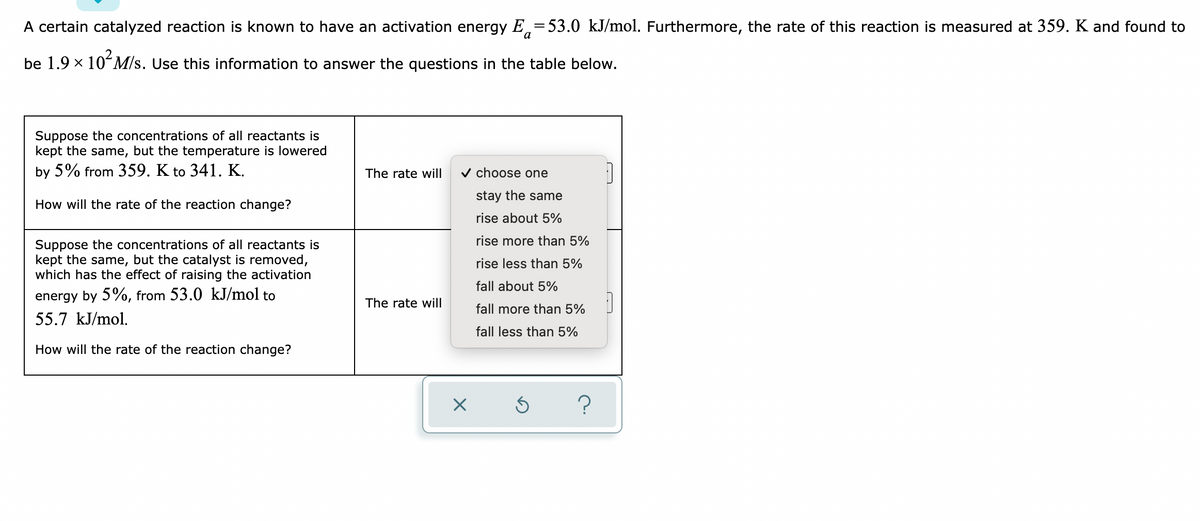
Transcribed Image Text:A certain catalyzed reaction is known to have an activation energy E = 53.0 kJ/mol. Furthermore, the rate of this reaction is measured at 359. K and found to
be 1.9 × 10² M/s. Use this information to answer the questions in the table below.
Suppose the concentrations of all reactants is
kept the same, but the temperature is lowered
by 5% from 359. K to 341. K.
The rate will
✓ choose one
stay the same
rise about 5%
How will the rate of the reaction change?
Suppose the concentrations of all reactants is
kept the same, but the catalyst is removed,
which has the effect of raising the activation
energy by 5%, from 53.0 kJ/mol to
55.7 kJ/mol.
The rate will
How will the rate of the reaction change?
X
rise more than 5%
rise less than 5%
fall about 5%
fall more than 5%
fall less than 5%
Ś
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning