Determine the heat transfer during this process.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
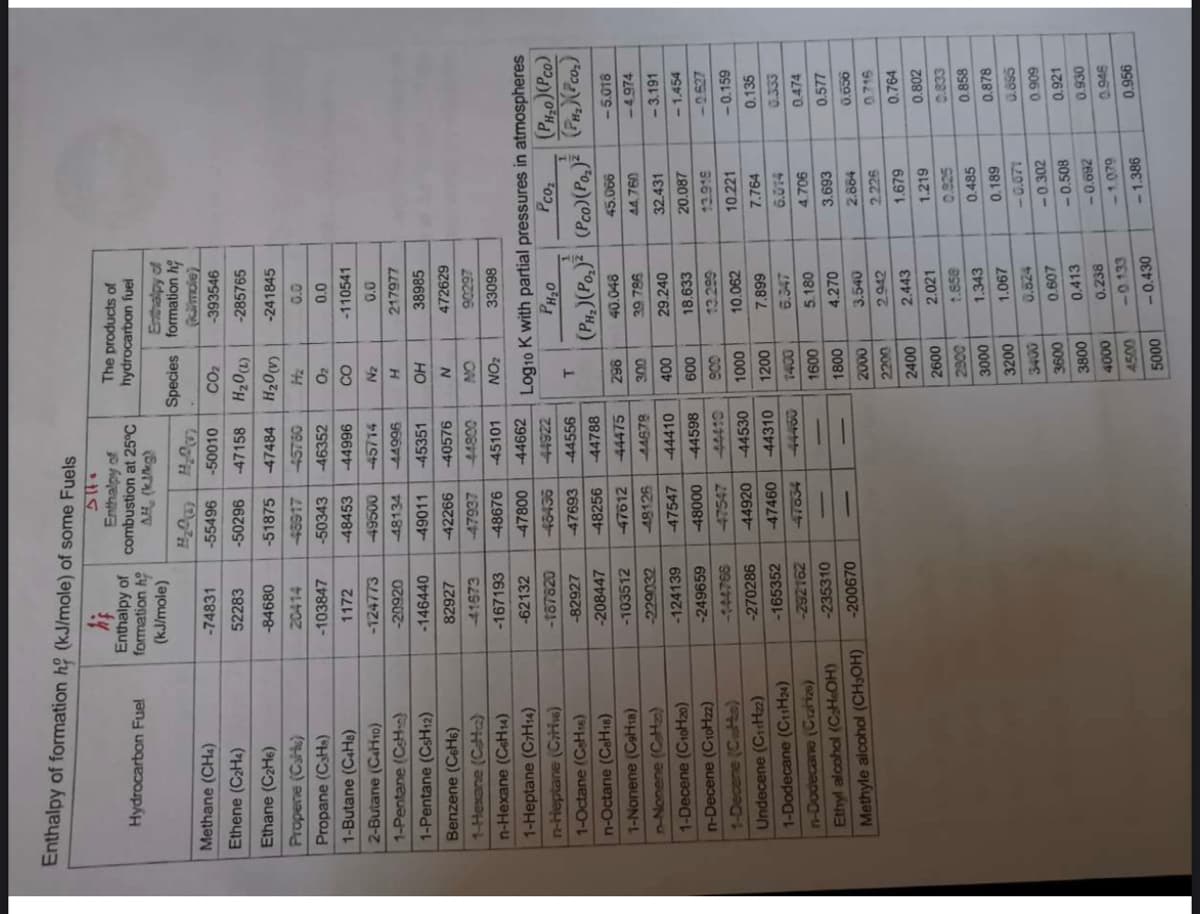
Transcribed Image Text:Enthalpy of formation hộ (kJ/mole) of some Fuels
Enthalpy of
formation h
(kJ/mole)
Enthalpy of
combustion at 25°C
The products of
hydrocarbon fuel
Hydrocarbon Fuel
AH, (KIMg)
Enthalpy of
Species formation h
mole)
Methane (CHa)
-74831
-55496
-50010
CO2
-393546
Ethene (C2H4)
52283
-50296
-47158
-285765
Ethane (C2H6)
-51875
-47484
089t8-
-241845
Propene (CaHo)
20414
Propane (CaH)
-103847
-50343
46352
O2
00
-110541
1-Butane (CaHs)
1172
-48453
2-Butane (CaH10)
966t-
-124773
0'0
217977
ZN
1-Pentane (CsH)
-20920
48134
H
966
1-Pentane (CsH12)
-146440
-49011
-45351
38985
HO
Benzene (CeH6)
82927
-42266
-40576
472629
1-Hexane (CaH)
41573
-47937
ON
0080
2606
n-Hexane (CeH14)
-167193
48676
860
Log10 K with partial pressures in atmospheres
Pcoz
O'H
(PH,)(Po,)| (Pco)(Po,)²
-45101
ZON
1-Heptane (CHs4)
-62132
47800
-44662
T-Heptane (CrtHivs)
43436
(PH,0)(Pco)
1-Octane (CeH1s)
-82927
-44556
n-Octane (CeH18)
-208447
-48256
44788
47612
44475
40.048
-5.018
(2 ) auauoN-L
-229032
-103512
967
990's
48126
44678
39 786
44.760
-4.974
-124139
-47547
-44410
29.240
32.431
- 3.191
1-Decene (C10H20)
-249659
-44598
0008-
18.633
20.087
- 1.454
n-Decene (C10H22)
009
-1:44768
47547
008
13 299
1-Decene (CaHe)
Undecene (C11Hz2)
-44920
-44530
000L
10.062
10.221
-0.159
-270286
7.764
0.135
-165352
-47460
-44310
1-Dodecane (CHH24)
668'2
6.014
47634
70L7SZ-
5.180
4.706
0.474
(aZLJ21) ueapog-u
-235310
Ethyl alcohol (CHOH)
009
4.270
3.693
0.577
-200670
008
Methyle alcohol (CH3OH)
3.540
000Z
0.716
2942
2226
1.679
0.764
2400
2.443
2.021
1.219
0.802
0097
0.925
1.658
0.485
0.858
1.343
000
0.878
0.189
1.067
606 0
0.921
0.607
-0.302
009
-0.508
0.413
008
0.930
Z690-
000
-0.133
-1.079
4500
0.956
- 1.386
-0.430
0009
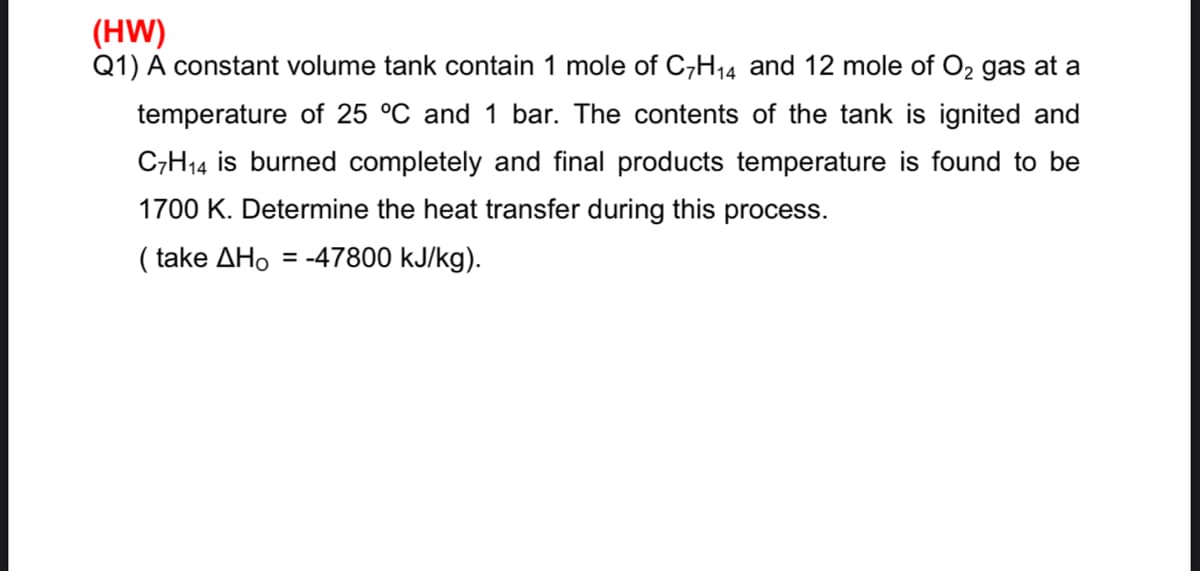
Transcribed Image Text:(HW)
Q1) A constant volume tank contain 1 mole of C,H14 and 12 mole of O2 gas at a
temperature of 25 °C and 1 bar. The contents of the tank is ignited and
C,H14 is burned completely and final products temperature is found to be
1700 K. Determine the heat transfer during this process.
( take ΔΗο
= -47800 kJ/kg).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY