Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter14: Acid-base Equilibria
Section: Chapter Questions
Problem 26E: Explain why the neutralization reaction of a strong acid and a weak base gives a weakly acidic...
Related questions
Question
Determine the nucleophile and electrophile in this reaction.
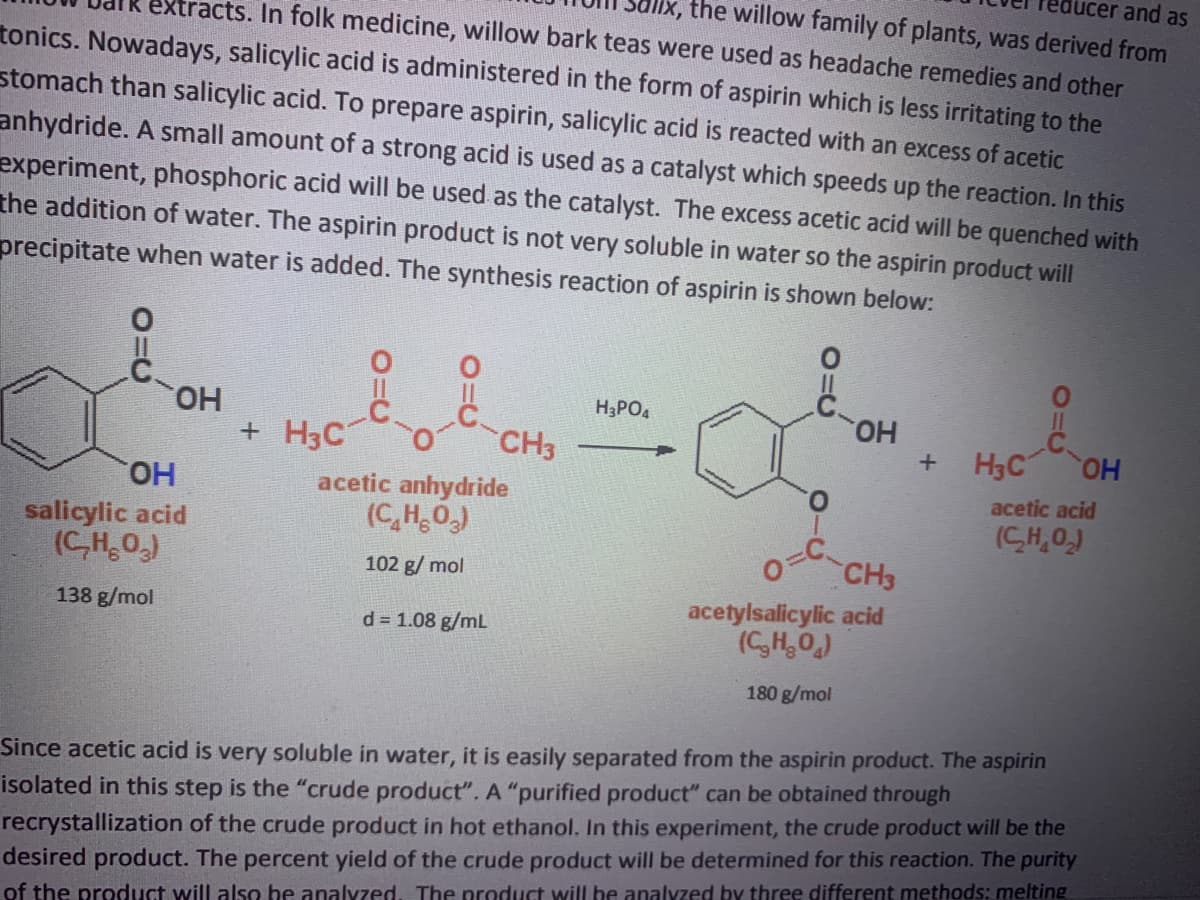
Transcribed Image Text:lucer and as
lx, the willow family of plants, was derived from
extracts. In folk medicine, willow bark teas were used as headache remedies and other
tonics. Nowadays, salicylic acid is administered in the form of aspirin which is less irritating to the
stomach than salicylic acid. To prepare aspirin, salicylic acid is reacted with an excess of acetic
anhydride. A small amount of a strong acid is used as a catalyst which speeds up the reaction. In this
experiment, phosphoric acid will be used as the catalyst. The excess acetic acid will be quenched with
the addition of water. The aspirin product is not very soluble in water so the aspirin product will
precipitate when water is added. The synthesis reaction of aspirin is shown below:
HO.
+ H3C
H3PO4
но.
H3C OH
CH3
HO.
salicylic acid
(C,H,O)
acetic anhydride
(C,H0,)
acetic acid
(CH,0,)
o=C
CH3
acetylsalicylic acid
(CH,0)
102 g/ mol
138 g/mol
d = 1.08 g/mL
180 g/mol
Since acetic acid is very soluble in water, it is easily separated from the aspirin product. The aspirin
isolated in this step is the "crude product". A "purified product" can be obtained through
recrystallization of the crude product in hot ethanol. In this experiment, the crude product will be the
desired product. The percent yield of the crude product will be determined for this reaction. The purity
of the product will also be analyzed, The product will be analyzed by three different methods: melting
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax