Determine the number of moles of oxygen atoms in each of the following. Part C 2.33x10-2 mol H2CO3 ? moles of oxygen = mol Part D 24.3 mol CO2 ? moles of oxygen = mol
Determine the number of moles of oxygen atoms in each of the following. Part C 2.33x10-2 mol H2CO3 ? moles of oxygen = mol Part D 24.3 mol CO2 ? moles of oxygen = mol
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 77E: Copper(I) iodide (CuI) is often added to table salt as a dietary source of iodine. How many moles of...
Related questions
Question
Please answer question 16 Part B, C, and D
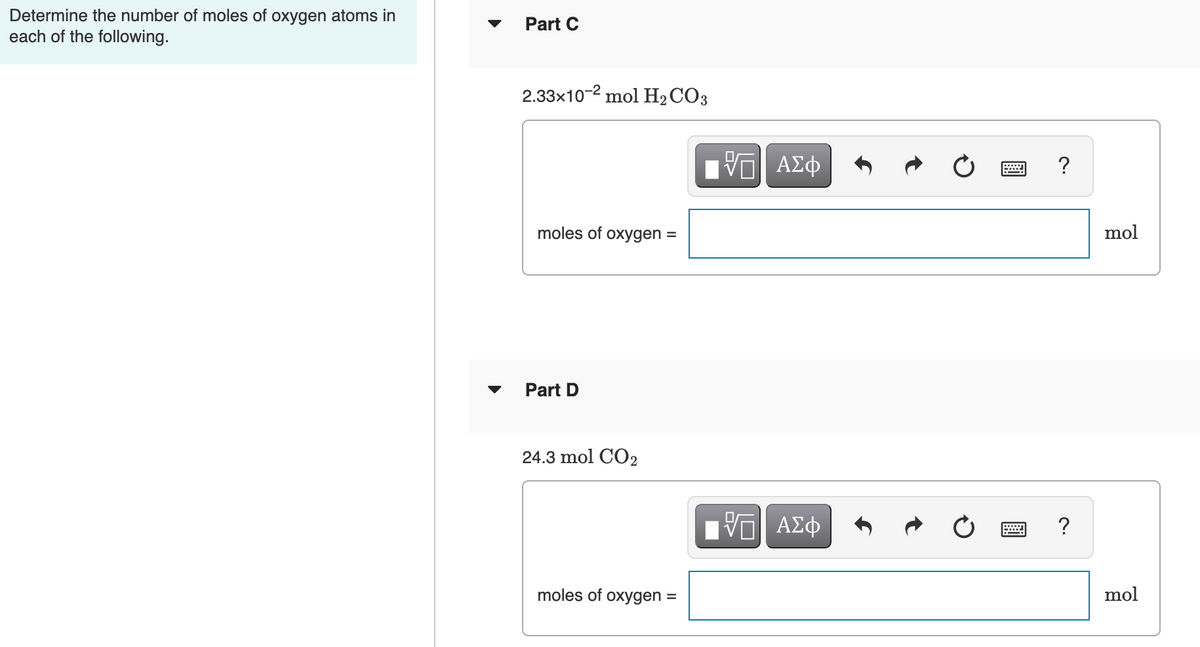
Transcribed Image Text:Determine the number of moles of oxygen atoms in
each of the following.
Part C
2.33x10-2 mol H2 CO3
HV ΑΣφ
?
moles of oxygen =
mol
Part D
24.3 mol CO2
ΠΠ ΑΣφ
?
moles of oxygen =
mol
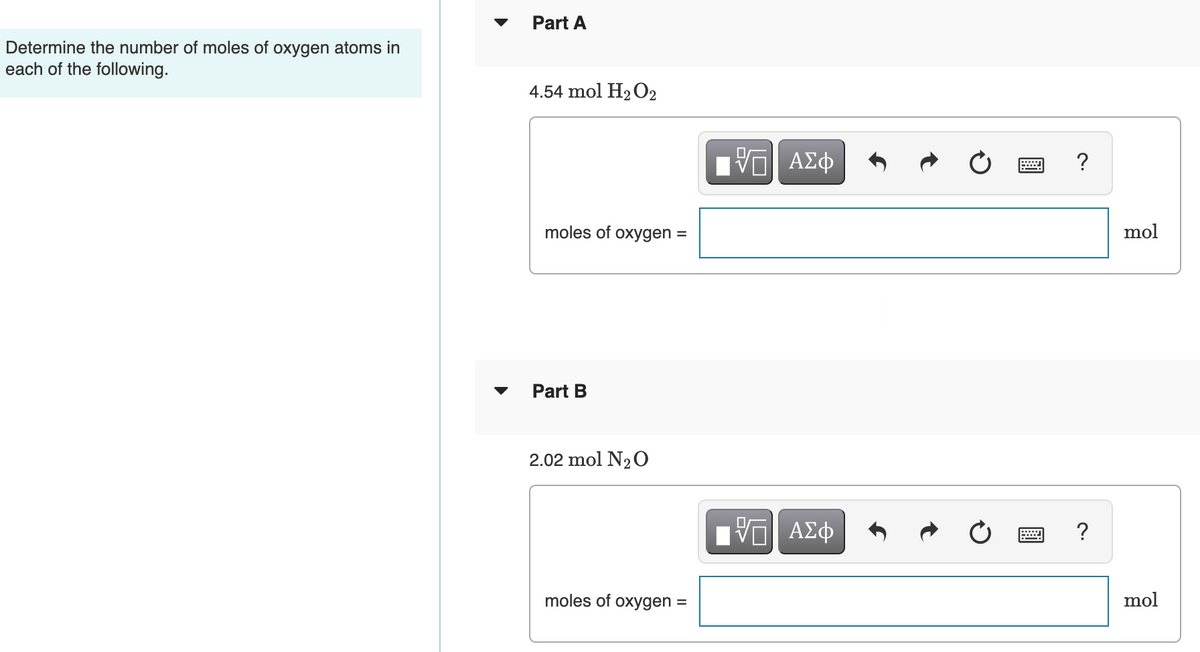
Transcribed Image Text:Part A
Determine the number of moles of oxygen atoms in
each of the following.
4.54 mol H2 O2
Bν ΑΣφ
?
moles of oxygen =
mol
Part B
2.02 mol N20
Hνα ΑΣφ
moles of oxygen =
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning