Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.88PAE: 4.88 A quality control technician needs to determine the percentage of arsenic (As) in a particular...
Related questions
Question
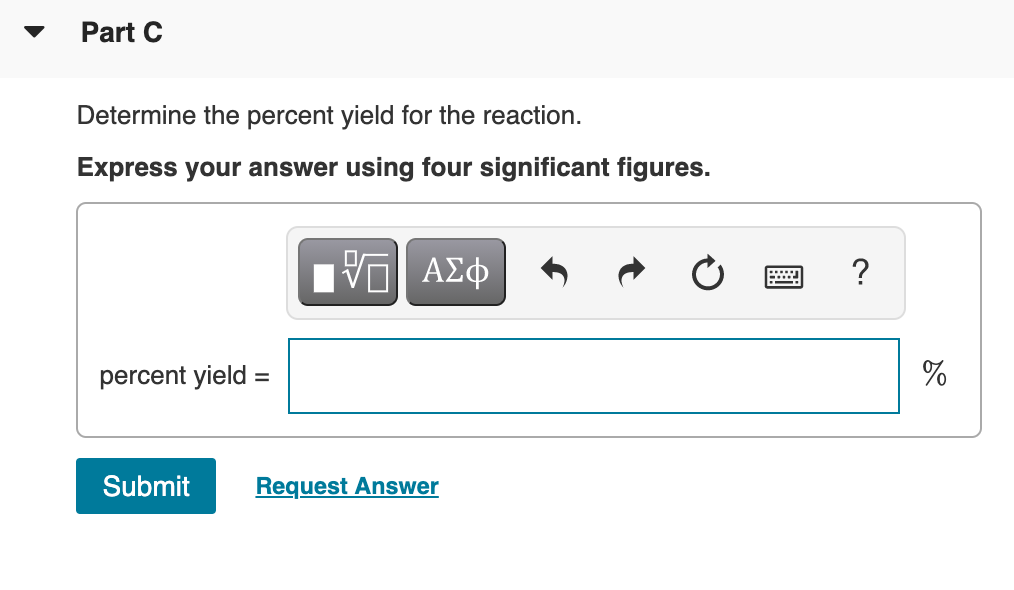
Transcribed Image Text:Part C
Determine the percent yield for the reaction.
Express your answer using four significant figures.
?
percent yield
Submit
Request Answer
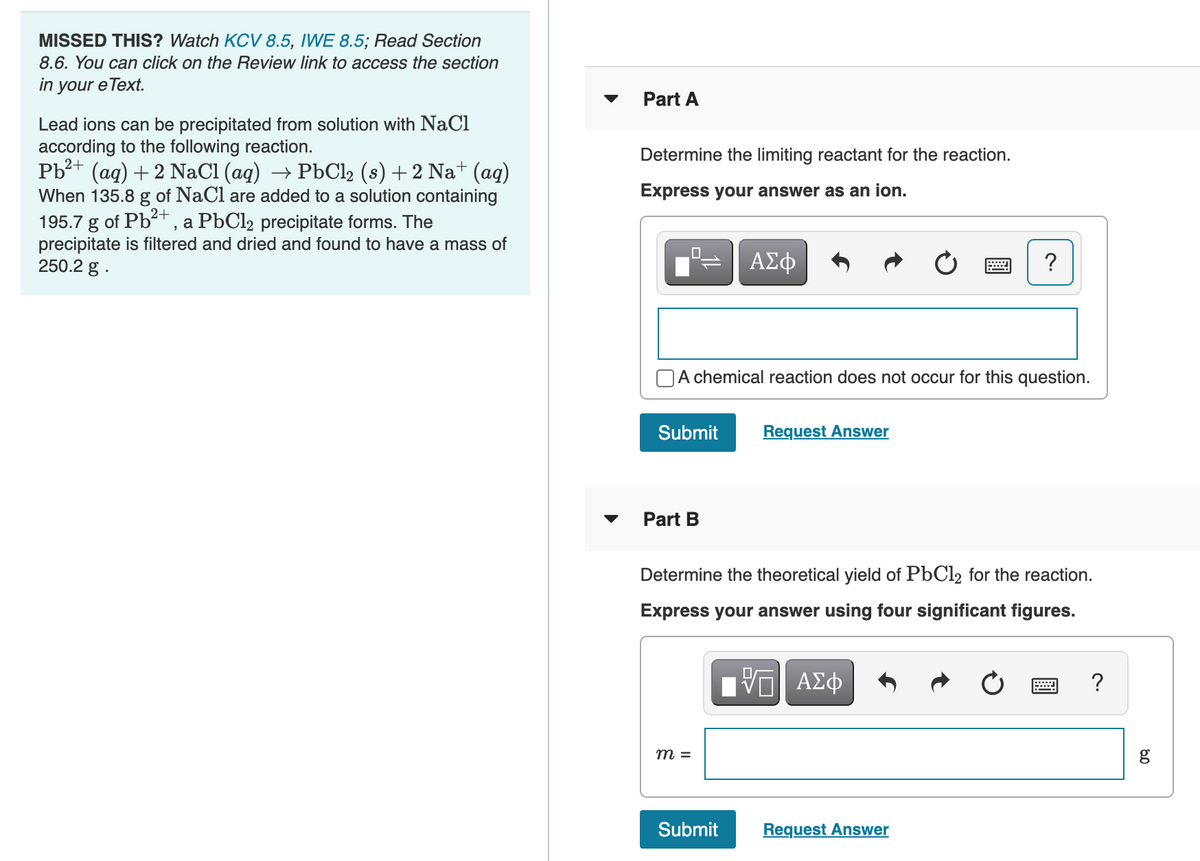
Transcribed Image Text:MISSED THIS? Watch KCV 8.5, IWE 8.5; Read Section
8.6. You can click on the Review link to access the section
in your e Text.
Part A
Lead ions can be precipitated from solution with NaCl
according to the following reaction.
Pb?+ (aq) + 2 NaCl (ag) → P6C12 (s) +2 Na+ (aq)
When 135.8 g of NaCl are added to a solution containing
195.7 g of Pb+ , a PbCl2 precipitate forms. The
precipitate is filtered and dried and found to have a mass of
250.2 g .
Determine the limiting reactant for the reaction.
Express your answer as an ion.
ΑΣφ
A chemical reaction does not occur for this question.
Submit
Request Answer
Part B
Determine the theoretical yield of PbCl2 for the reaction.
Express your answer using four significant figures.
Hν ΑΣφ
?
m =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning