Determine the pH of a solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. 3 NEXT > A solution is prepared with 0.075 M (CH3)³N and 0.10 M (CH3)NHCI. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) -2x 0.10-2x (CH3)3N(aq) + 0 0.075 + x 0.075 0.075-x 0.10 2 H₂O(l) 0.075 + 2x 5.2 x 10- 0.075 - 2x +x OH (aq) 0.10 + x -X 0.10-x + (CH,),NH*(aq) RESET +2x 0.10+2x
Determine the pH of a solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. 3 NEXT > A solution is prepared with 0.075 M (CH3)³N and 0.10 M (CH3)NHCI. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) -2x 0.10-2x (CH3)3N(aq) + 0 0.075 + x 0.075 0.075-x 0.10 2 H₂O(l) 0.075 + 2x 5.2 x 10- 0.075 - 2x +x OH (aq) 0.10 + x -X 0.10-x + (CH,),NH*(aq) RESET +2x 0.10+2x
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.1QAP
Related questions
Question
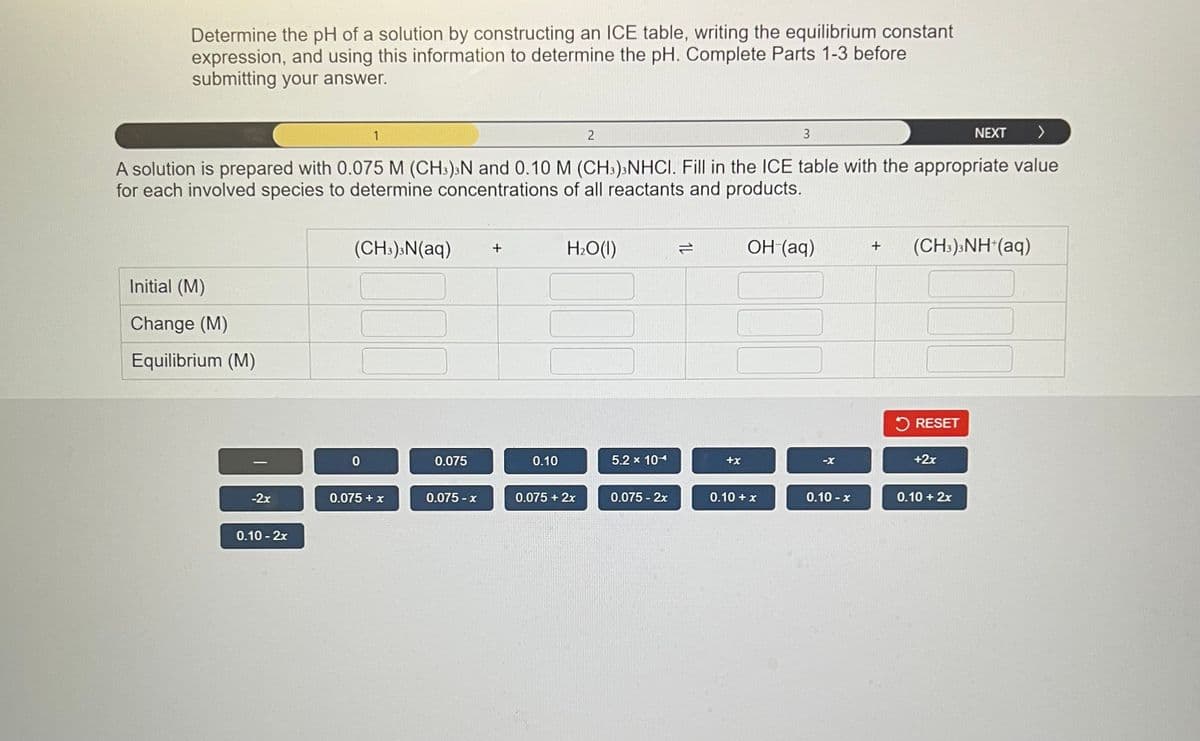
Transcribed Image Text:Determine the pH of a solution by constructing an ICE table, writing the equilibrium constant
expression, and using this information to determine the pH. Complete Parts 1-3 before
submitting your answer.
NEXT >
A solution is prepared with 0.075 M (CH3)3N and 0.10 M (CH3)3 NHCI. Fill in the ICE table with the appropriate value
for each involved species to determine concentrations of all reactants and products.
Initial (M)
Change (M)
Equilibrium (M)
-2x
0.10 - 2x
1
(CH3)3N(aq)
0
000
0.075 + x
0.075
0.075-x
+
0.10
2
H₂O(l)
0.075 + 2x
5.2 x 104
0.075 - 2x
1L
+x
3
OH (aq)
00
0.10 + x
0.10-x
+
(CH3)3NH+ (aq)
RESET
+2x
0.10 + 2x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

