Determine the solubility product constant (Ksp) of borax, a slightly soluble sodium salt, in water at two different temperatures and evaluate the enthalpy, entropy and Gibbs free energy change for dissolving borax in water.
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Determine the solubility product constant (Ksp) of borax, a slightly soluble sodium salt, in water at two different temperatures and evaluate the enthalpy, entropy and Gibbs free energy change for dissolving borax in water.
The equilibrium expression for the process is:
Na2B4O5(OH)4 10H2O (s) ⇌ 2 Na+ (aq) + B4O5(OH)4 2- (aq) + 10H2O(l)
Since liquid water and solid borax are not included in the Ksp expression, the solubility product expression associated with this reaction is:
Ksp = [Na+]2[borate]
The concentration of the borate ion in equilibrium with solid borax can be determined by titration with HCl based on the following equation:
B4O5(OH)42- + 2HCl(aq) + 3H2O (l) → 4 H3BO3(aq)+2Cl-(aq)
The Ksp for borax will be evaluated at room temperature at 5oC. Knowing the Ksp values at two temperatures allows the use of the following equations to calculate the change in enthalpy and Gibbs free energy:
In(Ksp1/Ksp2)=ΔH/R((1/T2)-(1/T1))
ΔGo= - RT InKsp
Once ΔHo and ΔGo are known, you can find ΔSo
ΔGo=ΔHo-TΔSo
Materials and reagents:
Borax (Na2B4O5(OH)4· 10H2O)
Na2CO3
0.1 M HCl solution
Bromocresol green indicator
Bromothymol blue indicator
500 ml Erlenmeyer flasks (10 per student)
Beakers (2 per student)
Magnetic stirrer and bar (1 per student)
Distilled water
Ice-water bath
Thermometer (1 per student)
50 ml burette (1 per student)
Procedure:
Preparation of the Borax-Borate Equilibrium Mixtures:
These solutions will be prepared beforehand.
Take about 60 mL of each solution. Make sure each student performs at least one titration.
- To two separate 500mL Erlenmeyer flasks containing magnetic stirring bars add about 20g of borax and 400mL of distilled water. Designate one flask as the room temperature system and one as the ice-water system.
- Stir the room temperature mixture gently on a magnetic stirrer (with no heating) for at least ten minutes. Shut off the stirrer, place a thermometer in the flask and leave it undisturbed to allow the excess undissolved borax to settle to the bottom.
- The solution portion should become clear after a few minutes. Place the second Erlenmeyer flask in an ice-water bath (be sure to use enough ice) on a magnetic stirrer and stir the mixture with no heating for at least 20 minutes.
- Shut off the stirrer, place a thermometer in the flask and allow the undissolved borax to settle to the bottom. This mixture temperature should be close to 5ºC and the solution portion should become clear. Keep the flask undisturbed in the ice bath until aliquots are removed for titration.
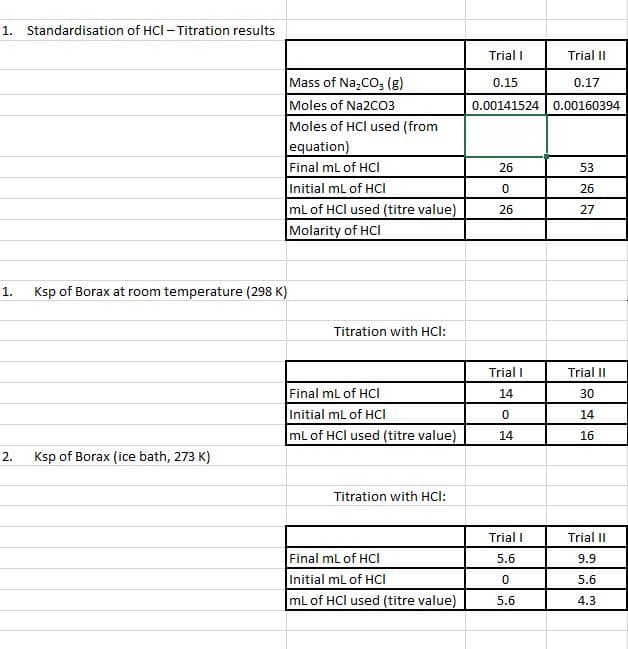
Standardization of 0.1 M HCl Titrating Solution:
- While the borax mixtures are stirring, standardize by titration using already prepared 0.1 M HCl. Use three 5-mL portions to rinse a clean 50 mL burette with the HCl solution and then fill the burette with the HCl solution.
- Accurately weigh three approximately 0.15 g portions of Na2CO3 to 0.01 g into separate clean but not necessarily dry Erlenmeyer flasks. To each flask add 3 drops of bromocresol green indicator and 50mL of distilled water. The solution will turn blue. Titrate each portion of sodium carbonate to the yellow-green endpoint.
- Do more than three titrations if your instructor says your results are not consistent. Use the titration data to calculate the actual concentration of your HCl solution according to the following equation:
Ksp of Borax at Room Temperature:
- Record the temperature of the room temperature borax mixture. Without disturbing the solid at the bottom, carefully decant about 60 mL of the borate solution into a clean and dry beaker and pipet three 10.00 mL aliquots into three separate clean but not necessarily dry Erlenmeyer flasks.
- Add 20 mL of distilled water and 3 drops of bromothymol blue indicator to each flask. The solutions should turn blue.
- Titrate each sample with your standardized HCl solution until the solution changes from blue to yellow-green. Use your best three titrations to evaluate the Ksp of borax at room temperature.
Ksp of Borax at Ice Temperature: Read the temperature of the iced borax mixture and carefully decant about 60 mL of the solution into a clean and dry beaker. Pipet three 10.00 mL aliquots into three separate Erlenmeyer flasks.
- To each flask, add 20 mL of distilled water and 3 drops of bromothymol blue indicator.
- Titrate each sample with HCl until the color changes to yellow-green.
- Use your best three titrations to evaluate the Ksp of borax at ice temperature.
![The chemical equation for the dissolution of borax may be written as:
Na, [B₂O(OH)4] - 8 H₂O(s) ⇒ 2 Na+ (aq) + B₂O(OH)²¯(aq) + 8 H₂O(1)
Tetraborate Anion
Borax
(11.5&
The tetraborate anion formed in solution is a weak base and reacts with water according to:
B₂O(OH)2 (aq) + 5 H₂O(aq) ⇒ 4H,BO₂(aq), OH¯(aq)
(11.6)
The products of the reaction are boric acid, H₂BO₂ (aq), a weak acid, and the hydroxide ion, OH (aq), a strong
base.
We can determine the concentration of the tetraborate anion in solution (and thus the amount of dissolved borax)
in the following way: First, we collect a sample of the saturated solution at equilibrium, being careful only to
collect the liquid portion and not any of the solid borax in equilibrium with it. Next we titrate the hydroxide ions
using a strong acid, say HCl (aq). As the hydroxide ions are removed from solution by the acid, more tetraborate
anions are converted into boric acid and hydroxide ions, in accordance with Le Chatelier's principle. That is, as
OH ions are removed from solution by the strong acid, the reaction described by Equation 11.6 will shift right.
We can represent the overall reaction between the tetraborate anions and the hydronium ions formed from the
strong acid, H₂O+ (aq), that occurs during the titration
BO(OH)²(aq) + 2 H₂O¹(aq)†H₂O(1) ⇒ 4H,BO₂ (aq)
(11.7)
The reaction described by Equation 11.7 will continue until we reach the equivalence point of the titration. At this
point all of the tetraborate anions have been converted to boric acid and all of the OH ions in the solution have
been neutralized. Because all of the base has been neutralized the addition of more strong acid causes the pH of
the solution to shifts sharply from basic to acidic. The presence of an acid-base indicator allows us to observe this
shift and will serve to signal the endpoint of our titration. The indicator we will use for this titration is bromocresol
green, which changes color from blue to yellow at a pH of 4.8 upon the addition of acid.
From the amount of acid required to reach the equivalence point, we can determine the concentration of
tetraborate anion present in the sample of our saturated borax solution. From this concentration we can
determine the value of Ksp for borax using a method analogous to that described previously for the dissolution of
Mg(OH)₂ (s). This is the method you will use in this experiment to determine the value of Ksp for borax.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff38eddb2-9ba1-4c72-995f-201ad019c840%2F36e42fd1-7748-45e0-a41a-079dea3709db%2Fv0c1abf_processed.jpeg&w=3840&q=75)
Trending now
This is a popular solution!
Step by step
Solved in 9 steps


