Determine the value of Ksp for Zn3(PO4)2 by constructing an ICE table, writing the solubility constant expression, and solving the expression. Complete Parts 1-2 before submitting your answer. NEXT > The molar solubility of Zn3(PO4)2 is 5.6 x 10-5 M at a certain temperature. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products at this temperature. Initial (M) Change (M) Equilibrium (M) 1.7 x 10-13 -1.12 x 10-5 0 1.9 x 10-8 1.68 x 10-5 Zn3(PO4)2(S) 5.6 x 10-5 5.9 x 10-20 -1.68 x 10-5 -5.6 x 10-5 -5.6 x 10-20 3 Zn²+ (aq) 1.12 x 10-4 2 -1.7 x 10-13 -1.12 x 10-4 -1.9 x 10-8 + 2 PO4³-(aq) 1.68 x 10-4 -5.9 x 10-20 RESET -1.68 x 10-4 1.12 x 10-5
Determine the value of Ksp for Zn3(PO4)2 by constructing an ICE table, writing the solubility constant expression, and solving the expression. Complete Parts 1-2 before submitting your answer. NEXT > The molar solubility of Zn3(PO4)2 is 5.6 x 10-5 M at a certain temperature. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products at this temperature. Initial (M) Change (M) Equilibrium (M) 1.7 x 10-13 -1.12 x 10-5 0 1.9 x 10-8 1.68 x 10-5 Zn3(PO4)2(S) 5.6 x 10-5 5.9 x 10-20 -1.68 x 10-5 -5.6 x 10-5 -5.6 x 10-20 3 Zn²+ (aq) 1.12 x 10-4 2 -1.7 x 10-13 -1.12 x 10-4 -1.9 x 10-8 + 2 PO4³-(aq) 1.68 x 10-4 -5.9 x 10-20 RESET -1.68 x 10-4 1.12 x 10-5
Chapter6: The Systematic Approach To Equilibria: Solving Many Equations
Section: Chapter Questions
Problem 11P
Related questions
Question
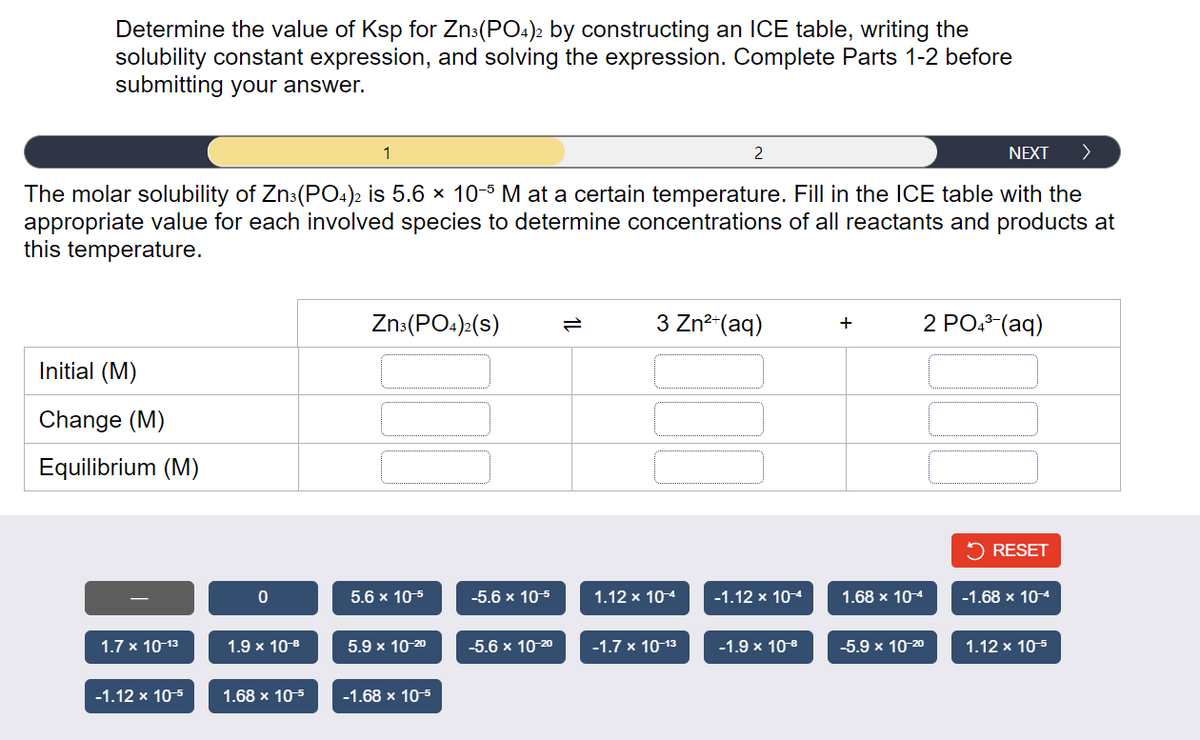
Transcribed Image Text:Determine the value of Ksp for Zn3(PO4)2 by constructing an ICE table, writing the
solubility constant expression, and solving the expression. Complete Parts 1-2 before
submitting your answer.
NEXT >
The molar solubility of Zn3(PO4)2 is 5.6 × 10-5 M at a certain temperature. Fill in the ICE table with the
appropriate value for each involved species to determine concentrations of all reactants and products at
this temperature.
Initial (M)
Change (M)
Equilibrium (M)
1.7 x 10-13
-1.12 x 10-5
0
1.9 x 10¹⁹
1.68 x 10-5
1
Zn3(PO4)2(S)
5.6 x 10-5
5.9 x 10-20
-1.68 x 10-5
-5.6 x 10-5
-5.6 x 10-2⁰
3 Zn²+ (aq)
1.12 x 10-4
2
-1.7 x 10-13
-1.12 x 10-4
-1.9 × 10-⁹
+
1.68 x 10-4
-5.9 x 10-20
2 PO4³-(aq)
RESET
-1.68 x 10-4
1.12 x 10-5
![Determine the value of Ksp for Zn³(PO4)2 by constructing an ICE table, writing the
solubility constant expression, and solving the expression. Complete Parts 1-2 before
submitting your answer.
PREV
Using the values from the ICE table, construct the expression for the solubility constant. Each reaction
participant must be represented by one tile. Do not combine terms Once the expression is constructed,
solve for Ksp.
[0]
[1.9 × 10-9]
[1.12 x 10-5]
[5.6 x 10-]
5.9 x 10-20
[1.68 x 10-⁹]
Ksp
1
[1.12 x 10-4]
[1.7 x 10-¹³1²
[1.12 x 10-1²
[1.68 x 10-4]
[1.9 × 10-1²
2.6 x 10-³
[5.6 x 10-51²
3.5x 10-39
=
3.8 x 10-4
2
[1.12 x 10-41²
[1.7 x 10-1³]³
[1.68 x 10-41³
[1.9 x 10-1³
✓ RESET
[1.7 x 10-¹³]
2.1 x 10-58](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F53fad175-5b35-4951-b14a-e374468693f0%2F2f1a94c6-0c06-419e-be22-7a9d7bc6a2ba%2F4wvuhdd_processed.png&w=3840&q=75)
Transcribed Image Text:Determine the value of Ksp for Zn³(PO4)2 by constructing an ICE table, writing the
solubility constant expression, and solving the expression. Complete Parts 1-2 before
submitting your answer.
PREV
Using the values from the ICE table, construct the expression for the solubility constant. Each reaction
participant must be represented by one tile. Do not combine terms Once the expression is constructed,
solve for Ksp.
[0]
[1.9 × 10-9]
[1.12 x 10-5]
[5.6 x 10-]
5.9 x 10-20
[1.68 x 10-⁹]
Ksp
1
[1.12 x 10-4]
[1.7 x 10-¹³1²
[1.12 x 10-1²
[1.68 x 10-4]
[1.9 × 10-1²
2.6 x 10-³
[5.6 x 10-51²
3.5x 10-39
=
3.8 x 10-4
2
[1.12 x 10-41²
[1.7 x 10-1³]³
[1.68 x 10-41³
[1.9 x 10-1³
✓ RESET
[1.7 x 10-¹³]
2.1 x 10-58
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

