Determine whether or not each of the following molecules is polar. For those that are polar, indicate the direction of the net dipole moment. (a) F (b) (d) CI (e) CI- F CI- -CI -CH3 Br F F (f) (g) CI (h) CI (i) Br Br Br CI G) (k) (1) (m) CI (n) Br CI CI CI CI \Br CI CI CI CI CI
Determine whether or not each of the following molecules is polar. For those that are polar, indicate the direction of the net dipole moment. (a) F (b) (d) CI (e) CI- F CI- -CI -CH3 Br F F (f) (g) CI (h) CI (i) Br Br Br CI G) (k) (1) (m) CI (n) Br CI CI CI CI \Br CI CI CI CI CI
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 6CTQ
Related questions
Question
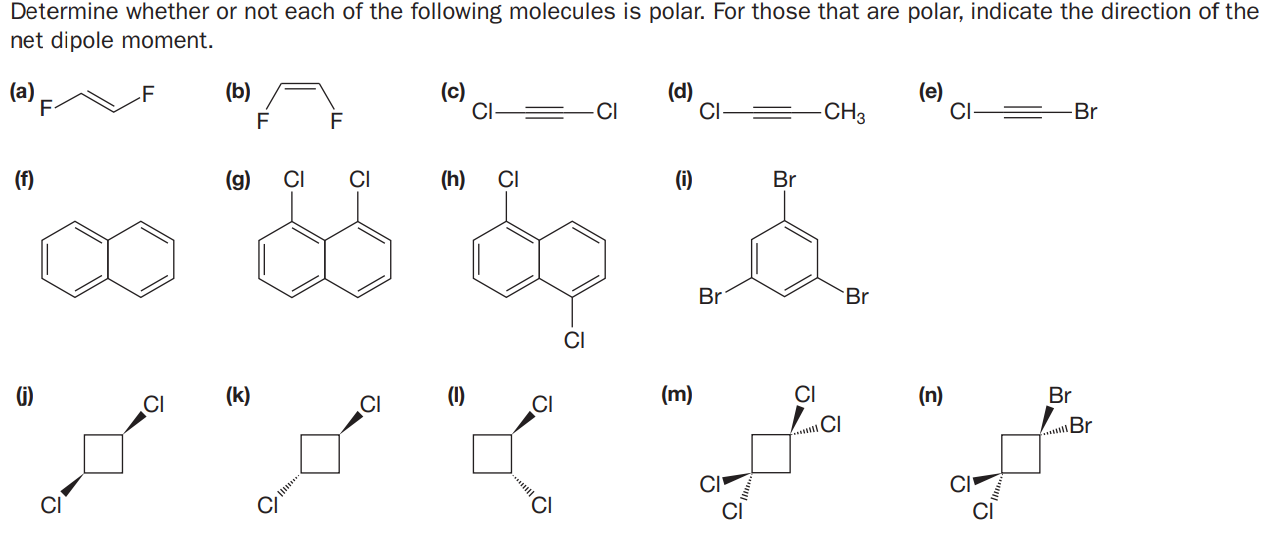
Transcribed Image Text:Determine whether or not each of the following molecules is polar. For those that are polar, indicate the direction of the
net dipole moment.
(a)
F
(b)
(d)
CI
(e)
CI-
F
CI-
-CI
-CH3
Br
F
F
(f)
(g)
CI
(h)
CI
(i)
Br
Br
Br
CI
G)
(k)
(1)
(m)
CI
(n)
Br
CI
CI
CI
CI
\Br
CI
CI
CI
CI
CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole