Dinitrogen tetraoxide is a colorless gas that dissociates into nitrogen dioxide, a reddish brown gas. N₂O4 (9) 2 NO₂ (g) An experiment was run to demonstrate that this is a dynamic equilibrium. Starting with a special form of N2O4 where one of the oxygens was isc labeled (180 instead of 160), the system was then allowed to reach equilibrium. O Indicate with Y (yes) or N (no) which of the substances below show that the forward or reverse reactions occur for this chemical system. A. O B. O O O O C. N D. 180 180
Dinitrogen tetraoxide is a colorless gas that dissociates into nitrogen dioxide, a reddish brown gas. N₂O4 (9) 2 NO₂ (g) An experiment was run to demonstrate that this is a dynamic equilibrium. Starting with a special form of N2O4 where one of the oxygens was isc labeled (180 instead of 160), the system was then allowed to reach equilibrium. O Indicate with Y (yes) or N (no) which of the substances below show that the forward or reverse reactions occur for this chemical system. A. O B. O O O O C. N D. 180 180
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter20: Electrochemistry
Section: Chapter Questions
Problem 14STP
Related questions
Question
Help on these two plz

Transcribed Image Text:Dinitrogen tetraoxide is a colorless gas that dissociates into nitrogen dioxide, a reddish brown gas.
N₂O4 (g) 2 NO₂ (g)
An experiment was run to demonstrate that this is a dynamic equilibrium. Starting with a special form of N₂O4 where one of the oxygens was isc
labeled (180 instead of 160), the system was then allowed to reach equilibrium.
O
Indicate with Y (yes) or N (no) which of the substances below show that the forward or reverse reactions occur for this chemical system.
A.
O
B.
O
O
O
O
C.
'N
D.
180
=
180
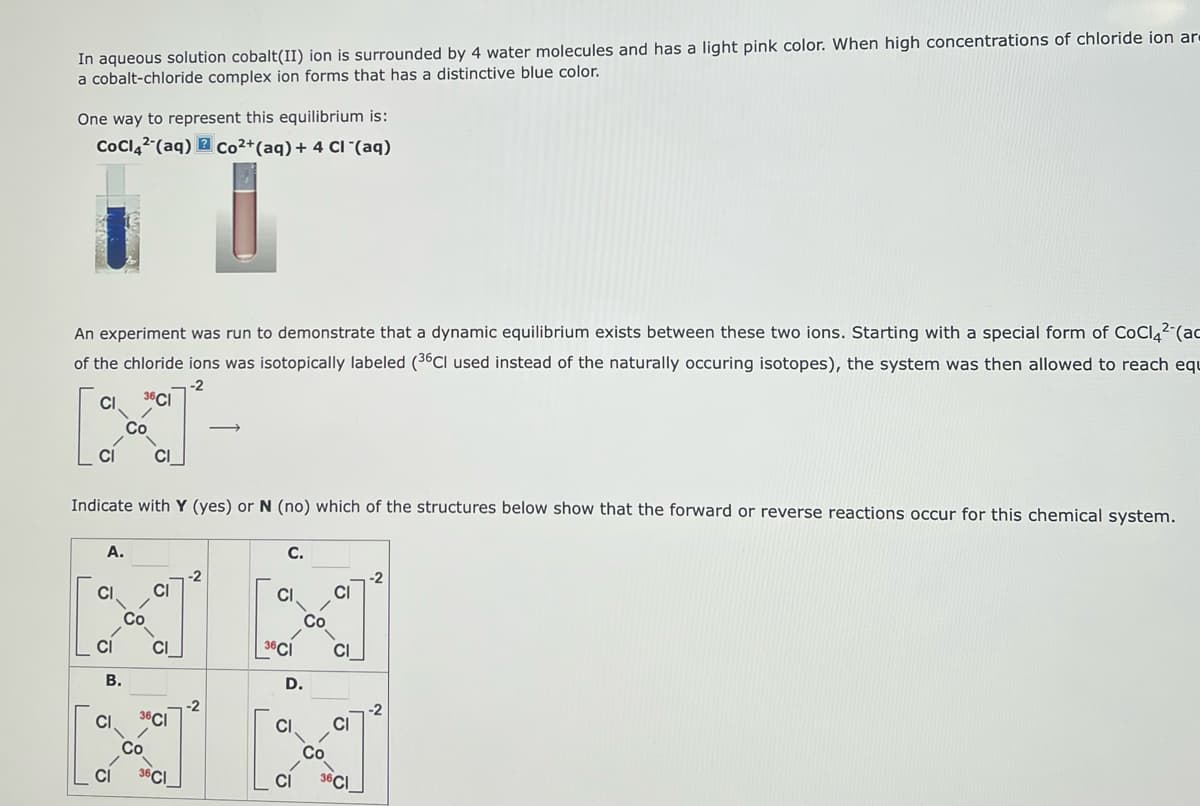
Transcribed Image Text:In aqueous solution cobalt(II) ion is surrounded by 4 water molecules and has a light pink color. When high concentrations of chloride ion ar-
a cobalt-chloride complex ion forms that has a distinctive blue color.
One way to represent this equilibrium is:
CoCl42 (aq) Co2+ (aq) + 4 CI (aq)
An experiment was run to demonstrate that a dynamic equilibrium exists between these two ions. Starting with a special form of CoCl42 (ad
of the chloride ions was isotopically labeled (36CI used instead of the naturally occuring isotopes), the system was then allowed to reach equ
-2
J
CI 36CI
Indicate with Y (yes) or N (no) which of the structures below show that the forward or reverse reactions occur for this chemical system.
A.
B.
CI
1.
CI
J
36CI
-ō
-2
-2
C.
-ان
36 CI
D.
CI
CI
Co
Co
CI
CI
G
36 CI
-2
Ń
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning