Direction: Complete the table by identifying the solute and solvent. Classify the types of solutions according to their phases: Solutions Components Solute Solvent Types of Solutions Gin Water & alcohol Steel Carbon & iron 18 Karat gold Gold & copper Soda Water & Carbon dioxide Water & ammonia Ammonia water Guide Questions: 1. How can you identify the solute and the solvent in a solution? 2. How will you classify solutions based on their phases?
Direction: Complete the table by identifying the solute and solvent. Classify the types of solutions according to their phases: Solutions Components Solute Solvent Types of Solutions Gin Water & alcohol Steel Carbon & iron 18 Karat gold Gold & copper Soda Water & Carbon dioxide Water & ammonia Ammonia water Guide Questions: 1. How can you identify the solute and the solvent in a solution? 2. How will you classify solutions based on their phases?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 68QAP: Consider two solutions at a certain temperature. Solution X has a nonelectrolyte as a solute and an...
Related questions
Question
100%
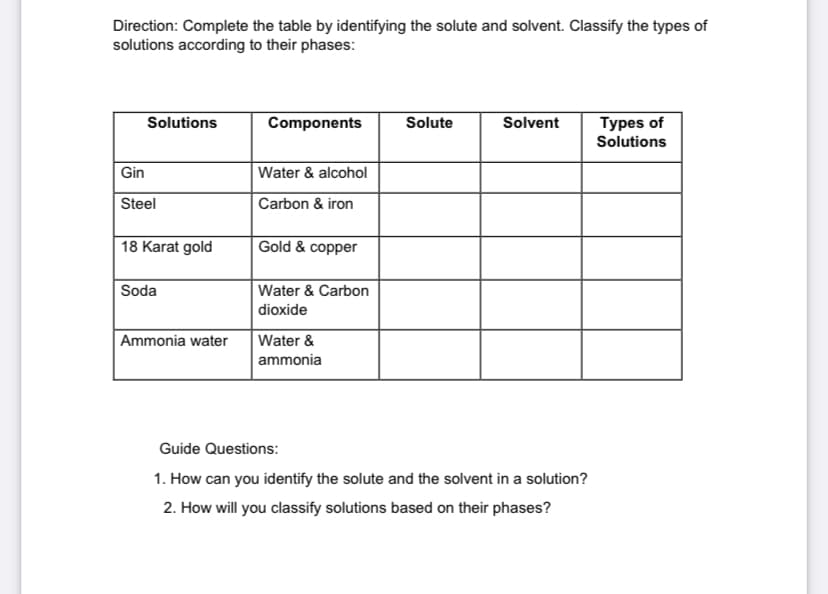
Transcribed Image Text:Direction: Complete the table by identifying the solute and solvent. Classify the types of
solutions according to their phases:
Types of
Solutions
Solutions
Components
Solute
Solvent
Gin
Water & alcohol
Steel
Carbon & iron
18 Karat gold
Gold & copper
Soda
Water & Carbon
dioxide
Ammonia water
Water &
ammonia
Guide Questions:
1. How can you identify the solute and the solvent in a solution?
2. How will you classify solutions based on their phases?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning