Directions: Write the letter of the word or phrase that best completes the statement or answers the question on the space provided. I. first order J. reaction order A. catalysts B. concentration C. rate law D. surface area E. temperature F. rate of reaction G. reactivity H. reactants 1. It refers to the amount of substance per unit volume. 2. Some reactions are slow and some are fast. 3. It tells the concentration dependence of one reactant in a reaction. 4. It is characterized as the tendency of a material to undergo a chemical reaction. 5. It shows the relationship between concentrations of reactants and reaction rate. 6. It describes the powers of the concentration factors in the rate equation. 7. It is a property of a material which increases as particle size decreases. 8. These are substances that go away with time in a reaction. 9. It increases the kinetic energy present in a material. 10. These are materials that do not change their amount throughout the chemical reaction.
Directions: Write the letter of the word or phrase that best completes the statement or answers the question on the space provided. I. first order J. reaction order A. catalysts B. concentration C. rate law D. surface area E. temperature F. rate of reaction G. reactivity H. reactants 1. It refers to the amount of substance per unit volume. 2. Some reactions are slow and some are fast. 3. It tells the concentration dependence of one reactant in a reaction. 4. It is characterized as the tendency of a material to undergo a chemical reaction. 5. It shows the relationship between concentrations of reactants and reaction rate. 6. It describes the powers of the concentration factors in the rate equation. 7. It is a property of a material which increases as particle size decreases. 8. These are substances that go away with time in a reaction. 9. It increases the kinetic energy present in a material. 10. These are materials that do not change their amount throughout the chemical reaction.
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 110CP: Consider the following hypothetical data collected in two studies of the reaction 2A+2BC+2D Time(s)...
Related questions
Question
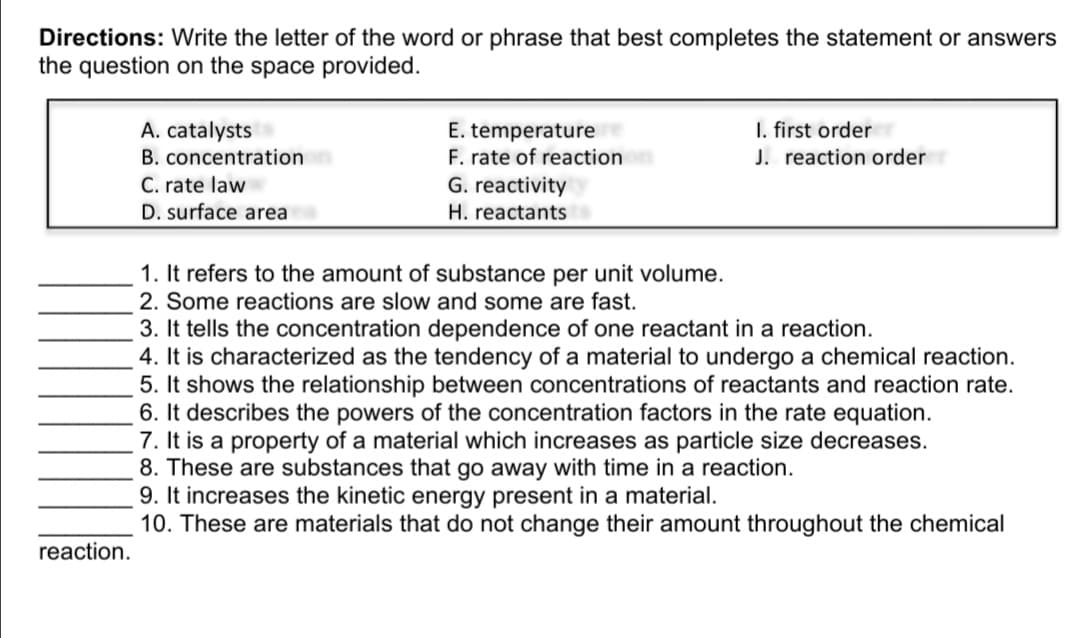
Transcribed Image Text:Directions: Write the letter of the word or phrase that best completes the statement or answers
the question on the space provided.
I. first order
J. reaction order
A. catalysts
E. temperature
F. rate of reaction
G. reactivity
B. concentration
C. rate law
D. surface area
H. reactants
1. It refers to the amount of substance per unit volume.
2. Some reactions are slow and some are fast.
3. It tells the concentration dependence of one reactant in a reaction.
4. It is characterized as the tendency of a material to undergo a chemical reaction.
5. It shows the relationship between concentrations of reactants and reaction rate.
6. It describes the powers of the concentration factors in the rate equation.
7. It is a property of a material which increases as particle size decreases.
8. These are substances that go away with time in a reaction.
9. It increases the kinetic energy present in a material.
10. These are materials that do not change their amount throughout the chemical
reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning