Distinguish between the equilibrium constant expression and Kp for the dissolution of a sparingly soluble salt. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the numerator. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the denominator. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the denominator. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the numerator. Submit Request Answer Next > Provide Feedback
Distinguish between the equilibrium constant expression and Kp for the dissolution of a sparingly soluble salt. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the numerator. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the denominator. The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration in the denominator. The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration in the numerator. Submit Request Answer Next > Provide Feedback
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 1.4ACP: Silver undergoes similar reactions as those shown for gold. Both metals react with cyanide ion in...
Related questions
Question
Distinguish between the equilibrium constant expression and the Ksp for the dissolution of a sparingly soluble salt.
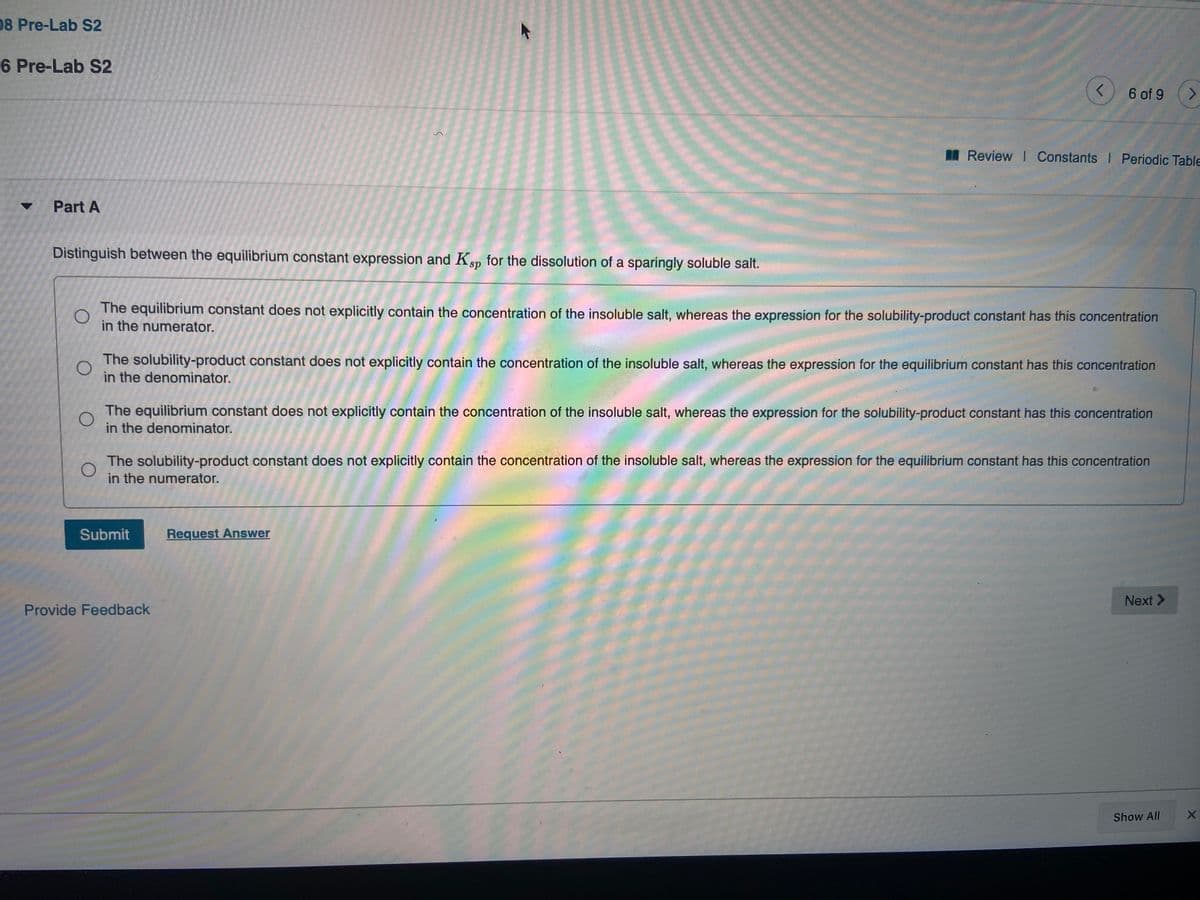
Transcribed Image Text:08 Pre-Lab S2
6 Pre-Lab S2
6 of 9
Review I Constants I Periodic Table
Part A
Distinguish between the equilibrium constant expression and Ksp for the dissolution of a sparingly soluble salt.
The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration
in the numerator.
The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration
in the denominator.
The equilibrium constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the solubility-product constant has this concentration
in the denominator.
The solubility-product constant does not explicitly contain the concentration of the insoluble salt, whereas the expression for the equilibrium constant has this concentration
in the numerator.
Submit
Request Answer
Next>
Provide Feedback
Show All
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
