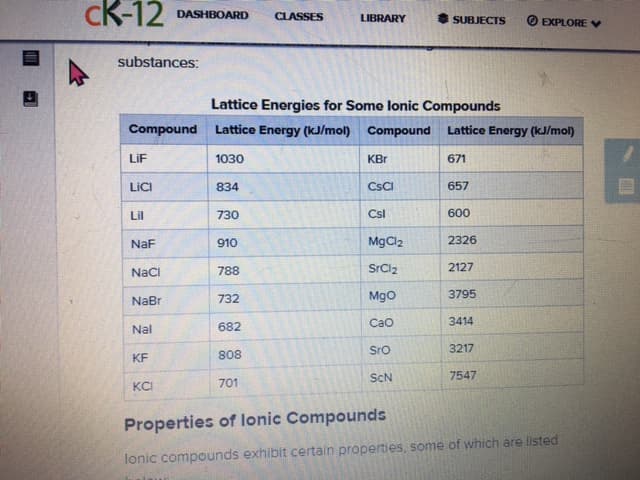
Does the lattice energy of an ionic solid compound increase or decreas as the charges of the ions increase, as the sixes of the ions increase? Arrange the following substances in tablattice energies, in table 8.2 according to their expected lattice energies, lowest to highest - MgS, Kl, GaN, LiBr
Does the lattice energy of an ionic solid compound increase or decreas as the charges of the ions increase, as the sixes of the ions increase? Arrange the following substances in tablattice energies, in table 8.2 according to their expected lattice energies, lowest to highest - MgS, Kl, GaN, LiBr
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section: Chapter Questions
Problem 16PS
Related questions
Question
Does the lattice energy of an ionic solid compound increase or decreas as the charges of the ions increase, as the sixes of the ions increase?
Arrange the following substances in tablattice energies, in table 8.2 according to their expected lattice energies, lowest to highest - MgS, Kl, GaN, LiBr
Transcribed Image Text:cK-12
DASHBOARD
CLASSES
LIBRARY
SUBJECTS
EXPLORE V
substances:
Lattice Energies for Some lonic Compounds
Compound
Lattice Energy (kJ/mol) Compound
Lattice Energy (kJ/mol)
LIF
1030
KBr
671
Lici
834
CSCI
657
Lil
730
Csl
600
NaF
910
MgCl2
2326
Nači
788
SrCl2
2127
NaBr
732
Mgo
3795
Cao
3414
Nal
682
Sro
3217
KF
808
ScN
7547
KCI
701
Properties of lonic Compounds
lonic compounds exhibit certain properties, some of which are listed
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax