8.83 Construct a Born-Haber cycle for the formation of the hy- pothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (a) How large would the lattice energy need to be for the formation of NaCl2 to be exothermic? (b) If we were to estimate the lattice energy of NaCl2 to be roughly equal to that of MgCl2 (2326 kJ/mol from Table 8.1), what value would you obtain for the standard enthalpy of formation, AH, of NaCl2? ing together of Na (g) and Cl (AH=-788 kJ/mol). Notice Table 8.1 lists the lattice energies for a number of ionic compounds. The large itive values indicate that the ions are strongly attracted to one another in ionic solid Pos TABLE 8.1 Lattice Energies for Some Ionic Compounds Lattice Energy (kJ/mol) Lattice Energy (kJ/mo Compound 2526 Compound MgCl2 1030 2127 LiF SrCl2 834 LiCl 730 Lil 3795 MgO 910 NaF 3414 CaO 788 NaCl 3217 SrO 732 NaBr 682 Nal ScN 7547 KF 808 КС. 701 KBr 671 CsCl 657 CsI 600
8.83 Construct a Born-Haber cycle for the formation of the hy- pothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (a) How large would the lattice energy need to be for the formation of NaCl2 to be exothermic? (b) If we were to estimate the lattice energy of NaCl2 to be roughly equal to that of MgCl2 (2326 kJ/mol from Table 8.1), what value would you obtain for the standard enthalpy of formation, AH, of NaCl2? ing together of Na (g) and Cl (AH=-788 kJ/mol). Notice Table 8.1 lists the lattice energies for a number of ionic compounds. The large itive values indicate that the ions are strongly attracted to one another in ionic solid Pos TABLE 8.1 Lattice Energies for Some Ionic Compounds Lattice Energy (kJ/mol) Lattice Energy (kJ/mo Compound 2526 Compound MgCl2 1030 2127 LiF SrCl2 834 LiCl 730 Lil 3795 MgO 910 NaF 3414 CaO 788 NaCl 3217 SrO 732 NaBr 682 Nal ScN 7547 KF 808 КС. 701 KBr 671 CsCl 657 CsI 600
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.74PAE: 7.74 In a lattice, a positive ion is often surrounded by eight negative ions. We might reason,...
Related questions
Question
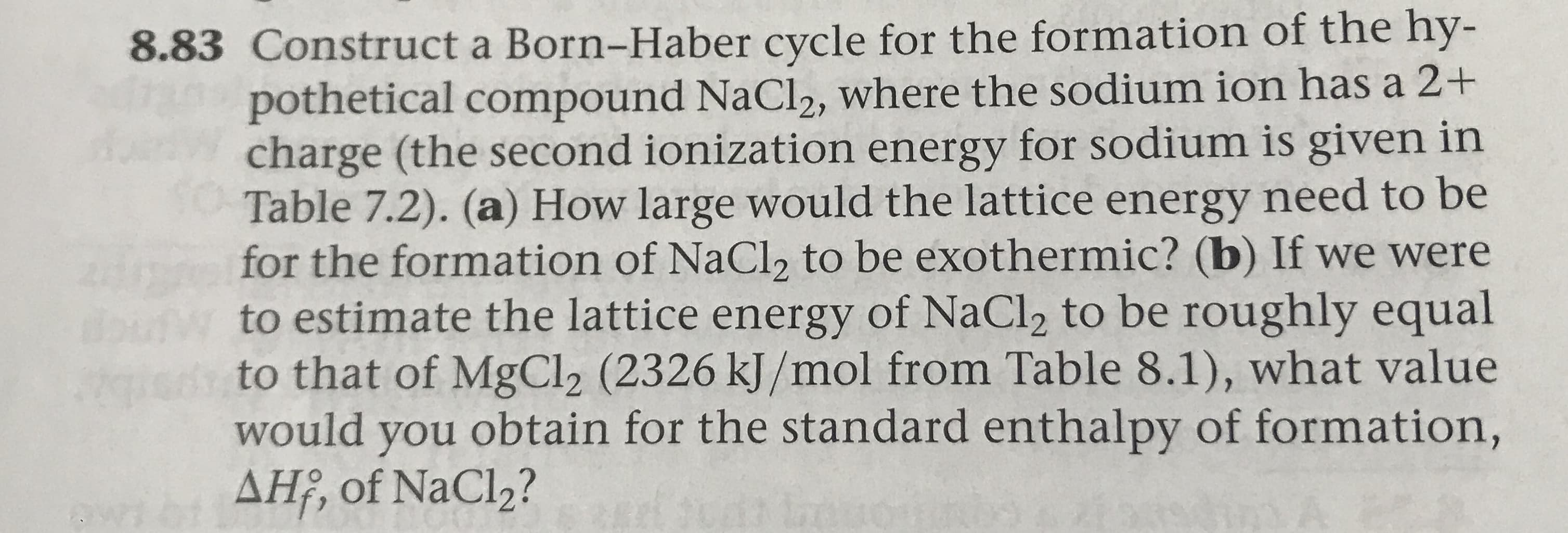
Transcribed Image Text:8.83 Construct a Born-Haber cycle for the formation of the hy-
pothetical compound NaCl2, where the sodium ion has a 2+
charge (the second ionization energy for sodium is given in
Table 7.2). (a) How large would the lattice energy need to be
for the formation of NaCl2 to be exothermic? (b) If we were
to estimate the lattice energy of NaCl2 to be roughly equal
to that of MgCl2 (2326 kJ/mol from Table 8.1), what value
would you obtain for the standard enthalpy of formation,
AH, of NaCl2?
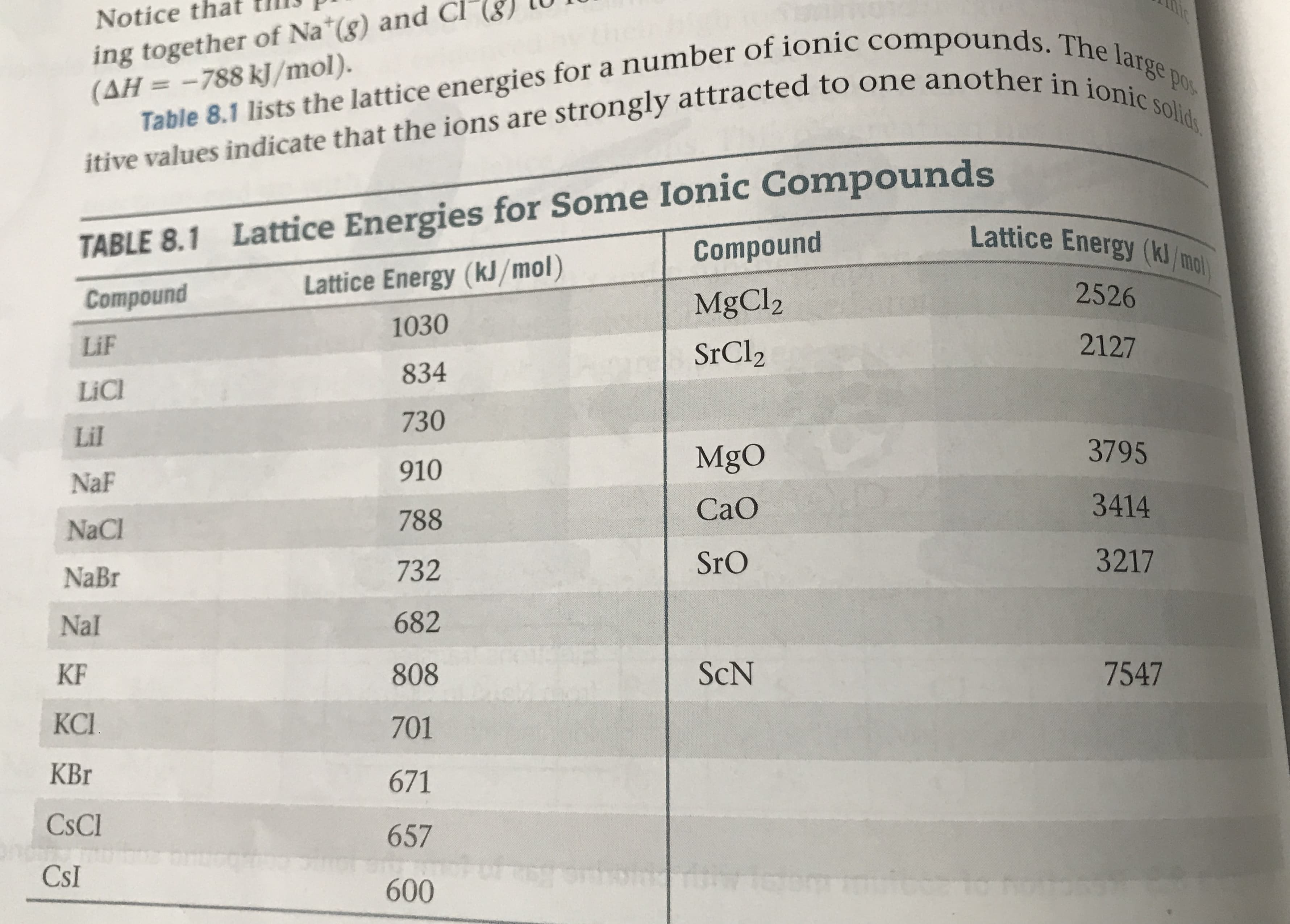
Transcribed Image Text:ing together of Na (g) and Cl
(AH=-788 kJ/mol).
Notice
Table 8.1 lists the lattice energies for a number of ionic compounds. The large
itive values indicate that the ions are strongly attracted to one another in ionic solid
Pos
TABLE 8.1 Lattice Energies for Some Ionic Compounds
Lattice Energy (kJ/mol)
Lattice Energy (kJ/mo
Compound
2526
Compound
MgCl2
1030
2127
LiF
SrCl2
834
LiCl
730
Lil
3795
MgO
910
NaF
3414
CaO
788
NaCl
3217
SrO
732
NaBr
682
Nal
ScN
7547
KF
808
КС.
701
KBr
671
CsCl
657
CsI
600
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning