Drag the appropriate items to their respective bins. Reset Help Attractive forces between atoms are present, but are not at maximum strength. The attractive forces between atoms are maximized resulting in the lowest energy state. The two atoms neither exert attractive nor repulsive forces on one another. Repulsive forces are high between the two atoms. A
Drag the appropriate items to their respective bins. Reset Help Attractive forces between atoms are present, but are not at maximum strength. The attractive forces between atoms are maximized resulting in the lowest energy state. The two atoms neither exert attractive nor repulsive forces on one another. Repulsive forces are high between the two atoms. A
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 67AP: The B2 molecule is paramagnetic; show how this indicates that the energy ordering of the orbitals in...
Related questions
Question
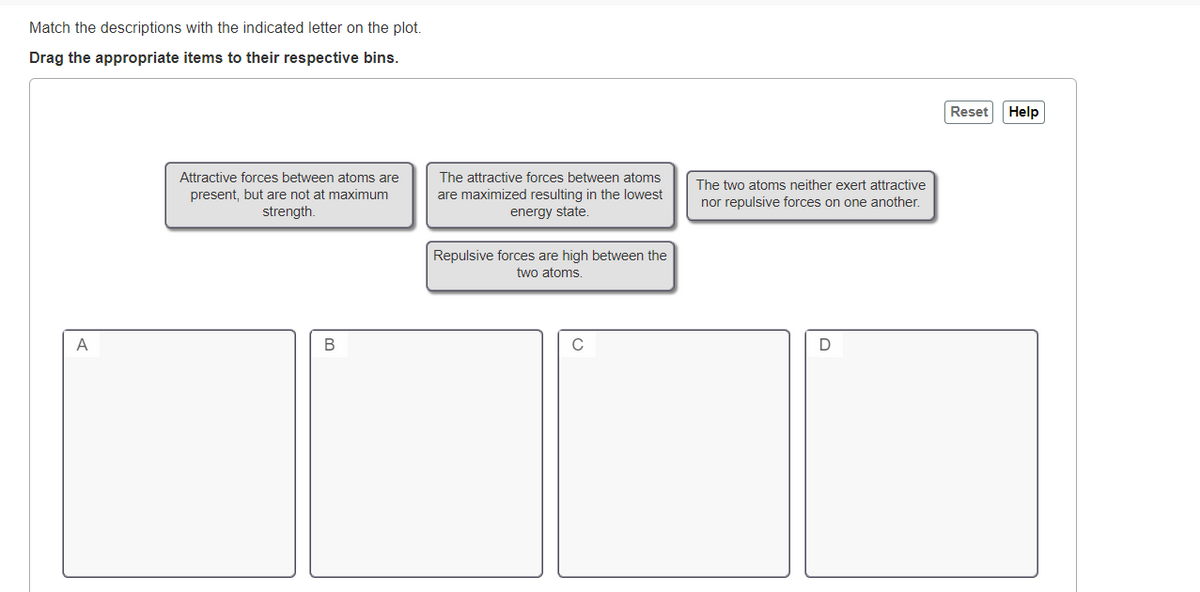
Transcribed Image Text:Match the descriptions with the indicated letter on the plot.
Drag the appropriate items to their respective bins.
Reset
Help
Attractive forces between atoms are
The attractive forces between atoms
The two atoms neither exert attractive
present, but are not at maximum
strength.
are maximized resulting in the lowest
energy state.
nor repulsive forces on one another.
Repulsive forces are high between the
two atoms.
A
B
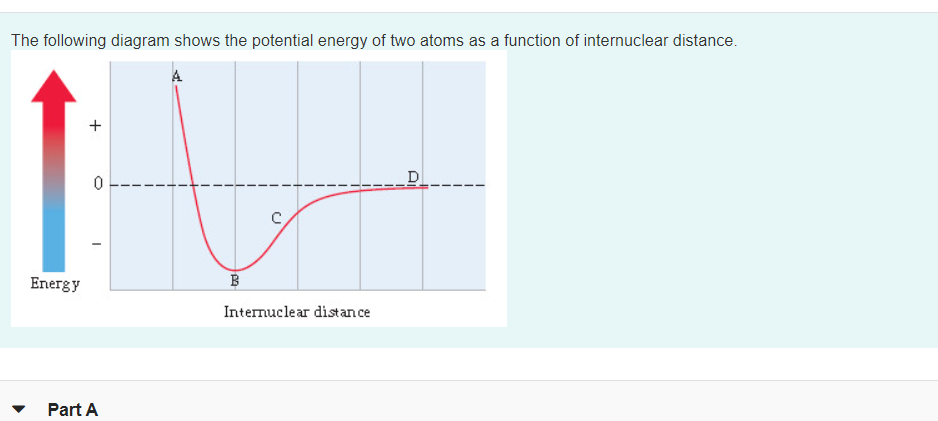
Transcribed Image Text:The following diagram shows the potential energy of two atoms as a function of internuclear distance.
D
Energy
B
Internuclear distance
Part A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER