Drag the tiles into the numerator or denominato to form the expression. Each reaction participant must be represented by one tile. Do not combine terms. Kp = Кр
Drag the tiles into the numerator or denominato to form the expression. Each reaction participant must be represented by one tile. Do not combine terms. Kp = Кр
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 83AE: Consider the following system at equilibrium at 25C: PCl3(g)+Cl(g)PCl5(g)G=92.50KJ What will happen...
Related questions
Question
Transcribed Image Text:6:06
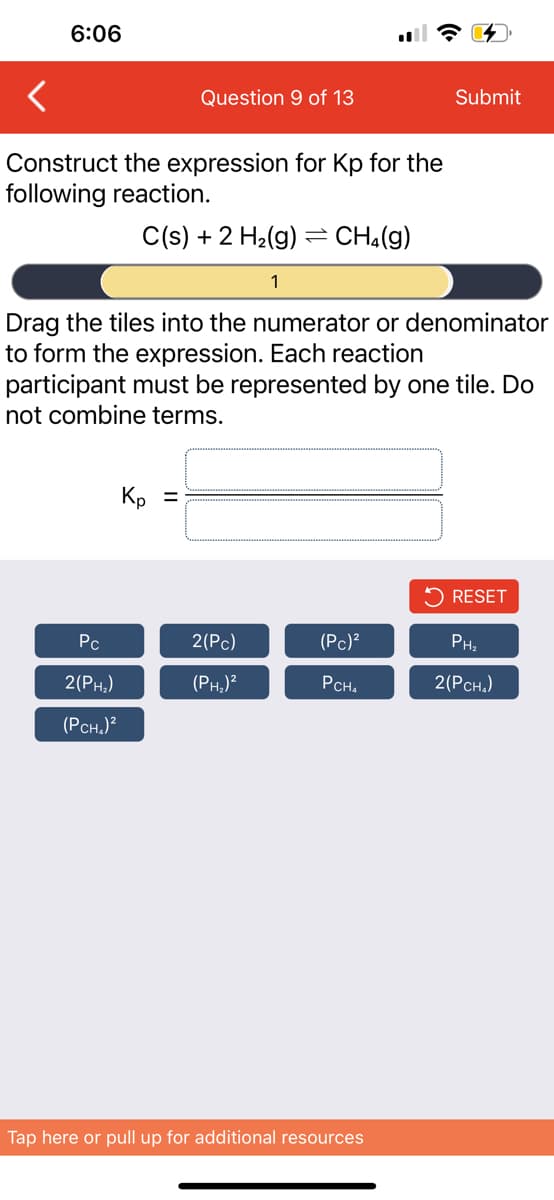
Construct the expression for Kp for the
following reaction.
C(s) + 2 H₂(g) = CH₂(g)
Pc
2
Question 9 of 13
Drag the tiles into the numerator or denominator
to form the expression. Each reaction
participant must be represented by one tile. Do
not combine terms.
2(PH₂)
(PCH₂)²
Kp =
1
2(Pc)
(PH₂)²
(Pc)²
PCH₂
Submit
Tap here or pull up for additional resources
RESET
PH₂
2(PCH₂)
Expert Solution
Step 1
Equilibrium constant at constant pressure, Kp is the product of the partial pressure of the products, each raised to the power equal to a stoichiometric coefficient divided by the product of the partial pressure of the reactants each raised to the power equal to its stoichiometric coefficient .
& the most important convention to be kept in mind is that the active mass of a pure solid or pure liquid is considered to be constant.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning