Draw a full orbital diagram for all the bonding and antibonding m orbitals in the three-membered cyclic cation shown here. Write your answers in the blank. CH How many pi electrons? BLANK open or close shell? BLANK aromatic or anti-aromatic? BLANK
Draw a full orbital diagram for all the bonding and antibonding m orbitals in the three-membered cyclic cation shown here. Write your answers in the blank. CH How many pi electrons? BLANK open or close shell? BLANK aromatic or anti-aromatic? BLANK
Chapter15: Benzene And Aromaticity
Section15.SE: Something Extra
Problem 53AP: Consider the aromatic anions below and their linear counterparts. Draw all of the resonance forms...
Related questions
Question
37
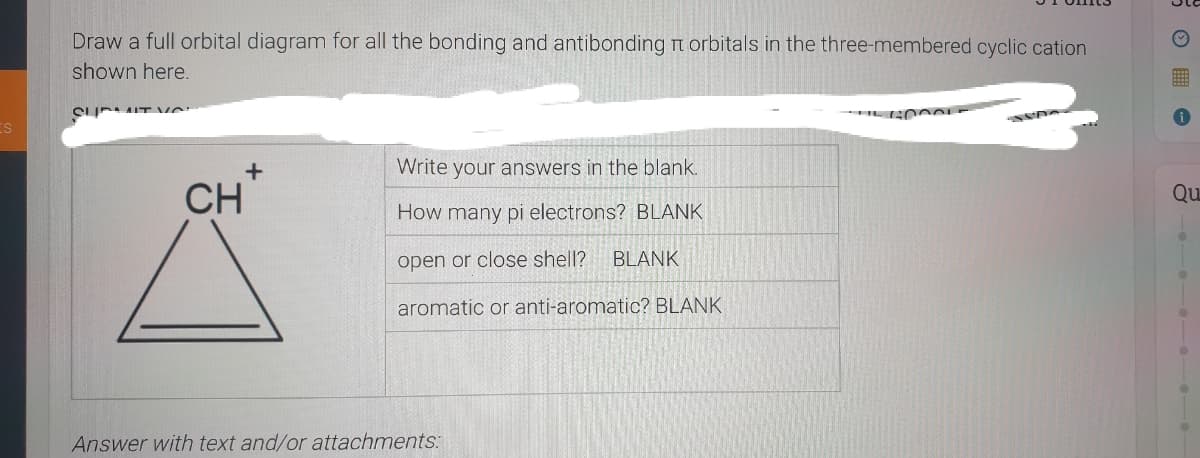
Transcribed Image Text:Draw a full orbital diagram for all the bonding and antibonding t orbitals in the three-membered cyclic cation
shown here.
CI IT ve
Write your answers in the blank.
CH
Qu
How many pi electrons? BLANK
open or close shell?
BLANK
aromatic or anti-aromatic? BLANK
Answer with text and/or attachments:
-e --
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

