A crucible and its cover weigh 65.82 grams. You add a hydrate of a salt and it now weighs 83.77 g heat the crucible, cover slightly ajar, until the contents are completely dry and it now weighs 81.06 9, 10. Calculate the experimental percent water in the sample in this hydrate. 11,12.Calculate the theoretical percent of water for the compound FeSO, 7H20
A crucible and its cover weigh 65.82 grams. You add a hydrate of a salt and it now weighs 83.77 g heat the crucible, cover slightly ajar, until the contents are completely dry and it now weighs 81.06 9, 10. Calculate the experimental percent water in the sample in this hydrate. 11,12.Calculate the theoretical percent of water for the compound FeSO, 7H20
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
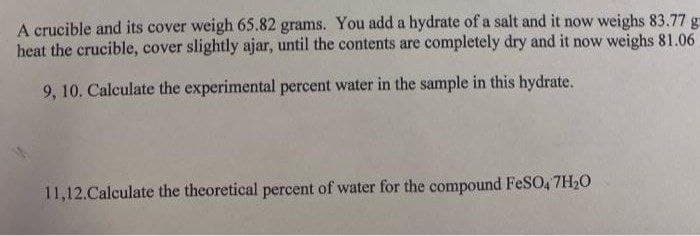
Transcribed Image Text:A crucible and its cover weigh 65.82 grams. You add a hydrate of a salt and it now weighs 83.77 g
heat the crucible, cover slightly ajar, until the contents are completely dry and it now weighs 81.06
9, 10. Calculate the experimental percent water in the sample in this hydrate.
11,12.Calculate the theoretical percent of water for the compound FeSO, 7H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



