Draw the Born-Haber cycle and use the data below for the formation of calcium chloride, to (ii) calculate the electron affinity of chlorine: RHat = +190 kJ/mol Ca(s) Ca(g) DHJ = +1730 kJ/mol 2e- Ca2* (9) + Ca(g) DHat = +121 kJ/mol Cl2(g) 2C(g) | Ca2* (g) CaCl2(s) DHLE = -2184 kJ/mol 2c(g) + DH = -795 kJ/mol Ca(s) + Cl2(g) CaCl2(s)
Draw the Born-Haber cycle and use the data below for the formation of calcium chloride, to (ii) calculate the electron affinity of chlorine: RHat = +190 kJ/mol Ca(s) Ca(g) DHJ = +1730 kJ/mol 2e- Ca2* (9) + Ca(g) DHat = +121 kJ/mol Cl2(g) 2C(g) | Ca2* (g) CaCl2(s) DHLE = -2184 kJ/mol 2c(g) + DH = -795 kJ/mol Ca(s) + Cl2(g) CaCl2(s)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 129QRT
Related questions
Question
100%
Please could someone help me with this question? I need it to be set out like the second image, but for CaCl2 and not MgCl2. Many thanks
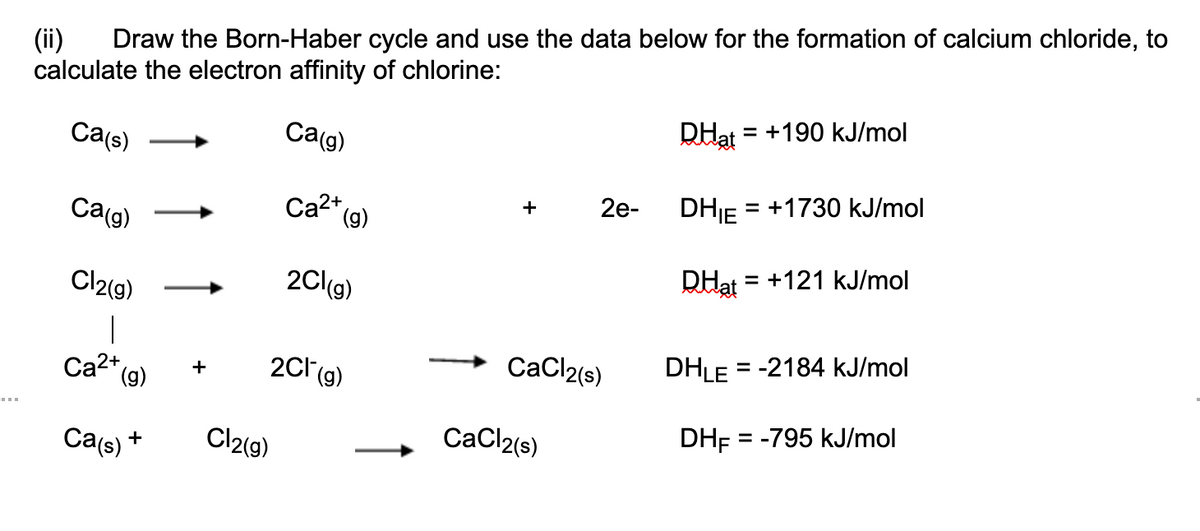
Transcribed Image Text:Draw the Born-Haber cycle and use the data below for the formation of calcium chloride, to
(ii)
calculate the electron affinity of chlorine:
DHạt
= +190 kJ/mol
Ca(g)
Ca(s)
2e-
DHJE = +1730 kJ/mol
Ca2* (g)
+
Ca(g)
DHạt = +121 kJ/mol
Cl2(g)
2Cl(g)
DHLE
= -2184 kJ/mol
Ca2* (g)
2C1 (9)
CaCl2(s)
+
CaCl2(s)
DHF = -795 kJ/mol
Ca(s)
Cl2(g)
+
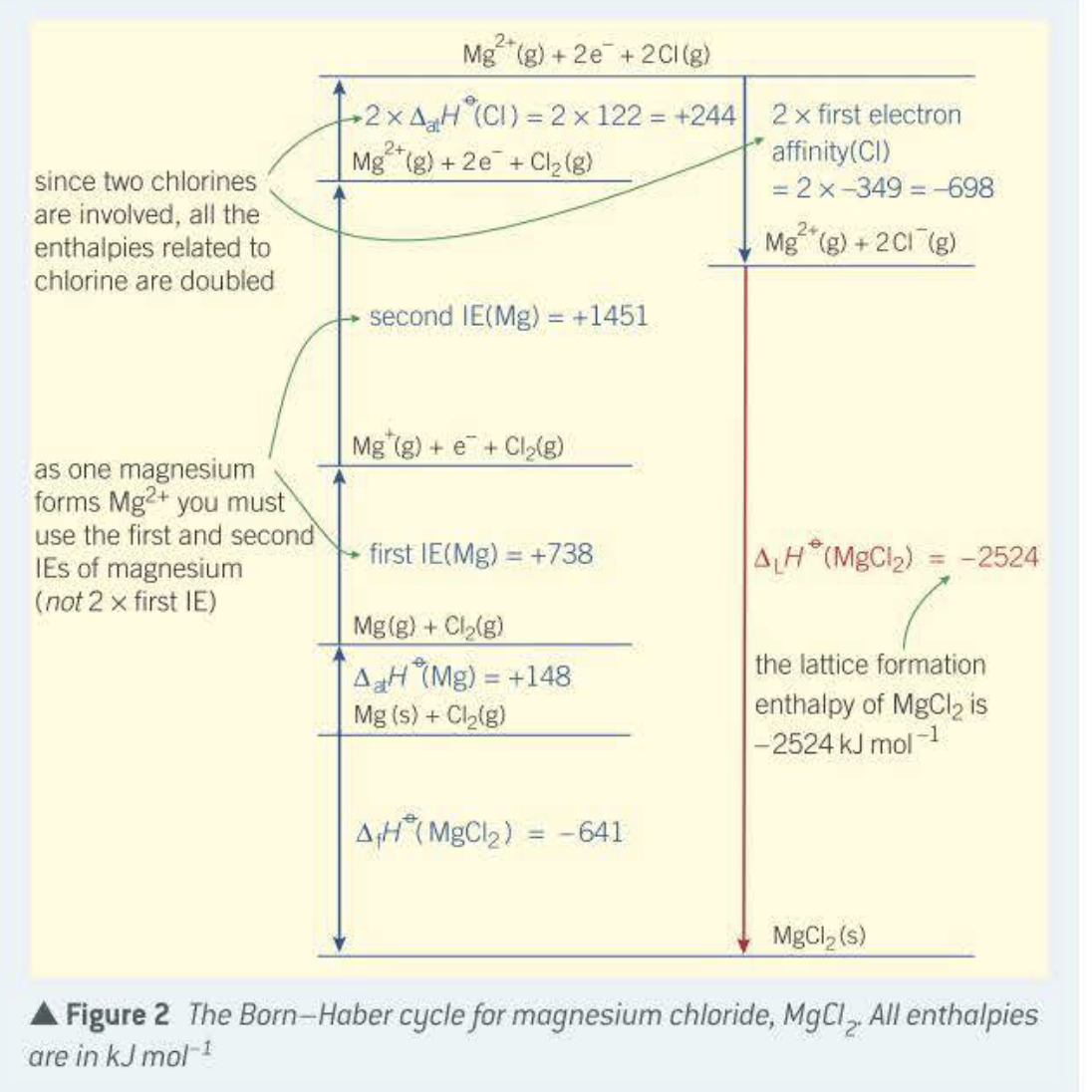
Transcribed Image Text:Mg (g) + 2e + 2CI(g)
2 x first electron
affinity(CI)
= 2x-349 = -698
2x AH (CI) = 2 x 122 = +244
%3D
%3D
2+
Mg (g) + 2e + Cl2 (g)
since two chlorines
are involved, all the
enthalpies related to
chlorine are doubled
24
Mg (g) + 2C1 (g)
second IE(Mg) = +1451
Mg (g) + e+ Cl(g)
as one magnesium
forms Mg2+ you must
use the first and second
IEs of magnesium
(not 2 x first IE)
first IE(Mg) = +738
ALH (MgCl2) = -2524
%3D
%3D
Mg(g) + Cl,(g)
the lattice formation
AH (Mg) = +148
Mg (s) + Clalg)
enthalpy of MgCl2 is
|-2524 kJ mol1
A;H (MgCl2)
= -641
MgCl2 (s)
Figure 2 The Born-Haber cycle for magnesium chloride, MgCl, All enthalpies
are in kJ mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co