Draw the curved arrow mechanism for the initiation step in cationic polymerization when methoxyethene reacts with BCI 3 H₂O. Include unshared electrons and any nonzero formal charges. You do not need to show additional resonance forms. K~]-[ i Draw the curved arrow mechanism for the initiation step of this polymerization reaction. ****** O +1: 12D :CI— CI: + H C N 0 S F Cl Br
Draw the curved arrow mechanism for the initiation step in cationic polymerization when methoxyethene reacts with BCI 3 H₂O. Include unshared electrons and any nonzero formal charges. You do not need to show additional resonance forms. K~]-[ i Draw the curved arrow mechanism for the initiation step of this polymerization reaction. ****** O +1: 12D :CI— CI: + H C N 0 S F Cl Br
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter29: Organic Polymer Chemistry
Section: Chapter Questions
Problem 29.22P: Draw a structural formula of the polymer resulting from base-catalyzed polymerization of each...
Related questions
Question
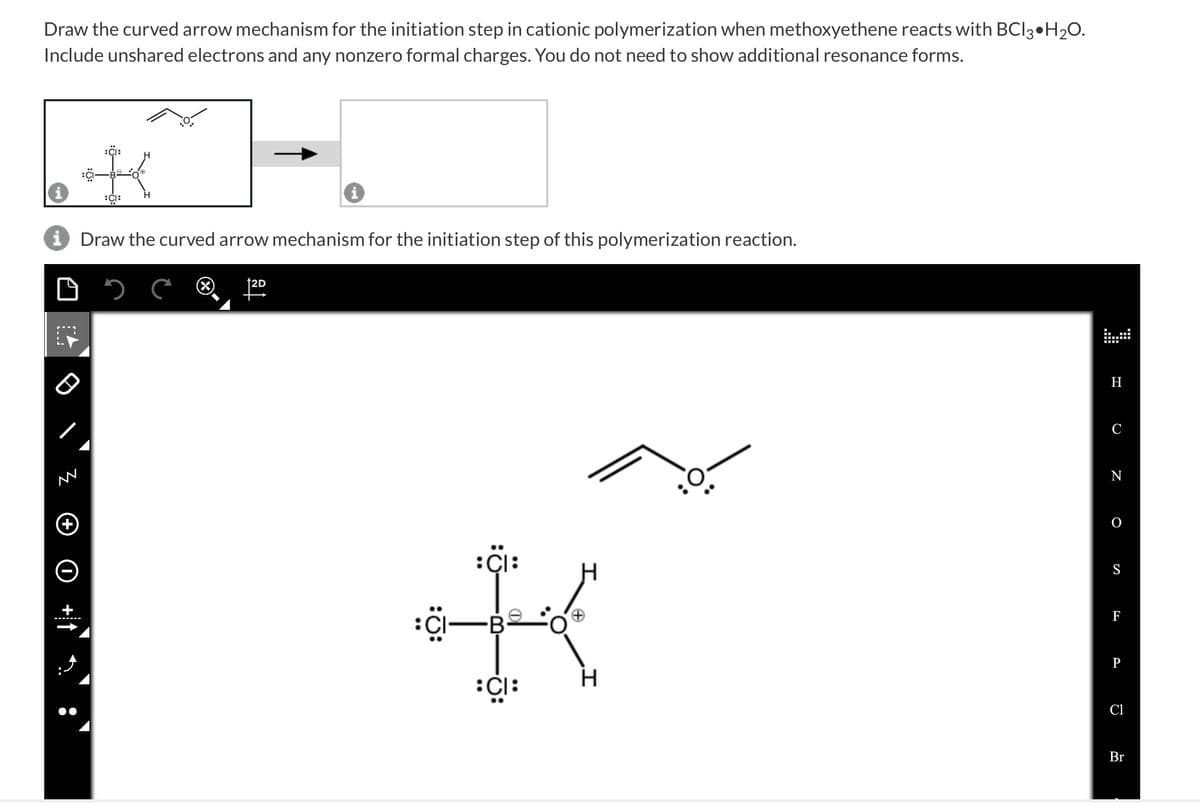
Transcribed Image Text:Draw the curved arrow mechanism for the initiation step in cationic polymerization when methoxyethene reacts with BCI3 H₂O.
Include unshared electrons and any nonzero formal charges. You do not need to show additional resonance forms.
TA
i Draw the curved arrow mechanism for the initiation step of this polymerization reaction.
□
AN
:CI:
‒‒‒‒‒‒‒
i
12D
: CI:
:CI—B
:CI:
+
I
H
C
N
O
S
F
P
Cl
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning