Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter21: Organic And Biological Molecules
Section: Chapter Questions
Problem 5RQ: What functional group distinguishes each of the following hydrocarbon derivatives? a....
Related questions
Question
100%
Draw the major product of the each of the reactions in the presence of acid and water and the oxidizing agent shown.
![Alcohols are a key intermediate in organic synthesis since they can oxidized to carbonyl containing functional
groups.
In this tutorial, we will cover:
1. what happens in an oxidation reaction
2. oxidation of secondary alcohols to ketones
3. oxidation of primary alcohols to aldehydes or carboxylic acids
4. oxidation reagents and how we can control oxidation
Oxidation reactions in organic chemistry have historically used chromium reagents for alcohol oxidation. In textbooks, these
are shown as Cr(VI), K,Cr,O,, Na, Cr,O,, or CrO3. Oxidation of secondary alcohols with chromium reagents are typically
carried out in aqueous acid, which convert the chromium reagent to chromic acid (H,CrO,). The mechanism can be thought
of as installing a good leaving group onto the oxygen. The key step is an elimination, where the hydrogen is abstracted,
forming a new T bond and kicking off the leaving group.
нӧ:
K2Cr207
-он
H.
Primary alcohols react under the same conditions to give carboxylic acids. Why does this occur? Primary alcohols will
undergo oxidation to an aldehyde, but the presence of the aqueous acid leads to formation of a diol, which can be further
oxidized to the carboxylic acid. The diol can undergo the same reaction with chromium and oxidation to remove
two hydrogens.
НО
НО
[0]
H20
OH
[0]
H.
H.
H.
HO.
H
Draw the major product of the each of the reactions in the presence of acid and water and the oxidizing agent shown.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3129c971-f425-413a-93d6-6547672e04e6%2F72a20bc7-c0ac-4975-a9e8-d7ac9d93d27d%2Fuc59jk9_processed.png&w=3840&q=75)
Transcribed Image Text:Alcohols are a key intermediate in organic synthesis since they can oxidized to carbonyl containing functional
groups.
In this tutorial, we will cover:
1. what happens in an oxidation reaction
2. oxidation of secondary alcohols to ketones
3. oxidation of primary alcohols to aldehydes or carboxylic acids
4. oxidation reagents and how we can control oxidation
Oxidation reactions in organic chemistry have historically used chromium reagents for alcohol oxidation. In textbooks, these
are shown as Cr(VI), K,Cr,O,, Na, Cr,O,, or CrO3. Oxidation of secondary alcohols with chromium reagents are typically
carried out in aqueous acid, which convert the chromium reagent to chromic acid (H,CrO,). The mechanism can be thought
of as installing a good leaving group onto the oxygen. The key step is an elimination, where the hydrogen is abstracted,
forming a new T bond and kicking off the leaving group.
нӧ:
K2Cr207
-он
H.
Primary alcohols react under the same conditions to give carboxylic acids. Why does this occur? Primary alcohols will
undergo oxidation to an aldehyde, but the presence of the aqueous acid leads to formation of a diol, which can be further
oxidized to the carboxylic acid. The diol can undergo the same reaction with chromium and oxidation to remove
two hydrogens.
НО
НО
[0]
H20
OH
[0]
H.
H.
H.
HO.
H
Draw the major product of the each of the reactions in the presence of acid and water and the oxidizing agent shown.
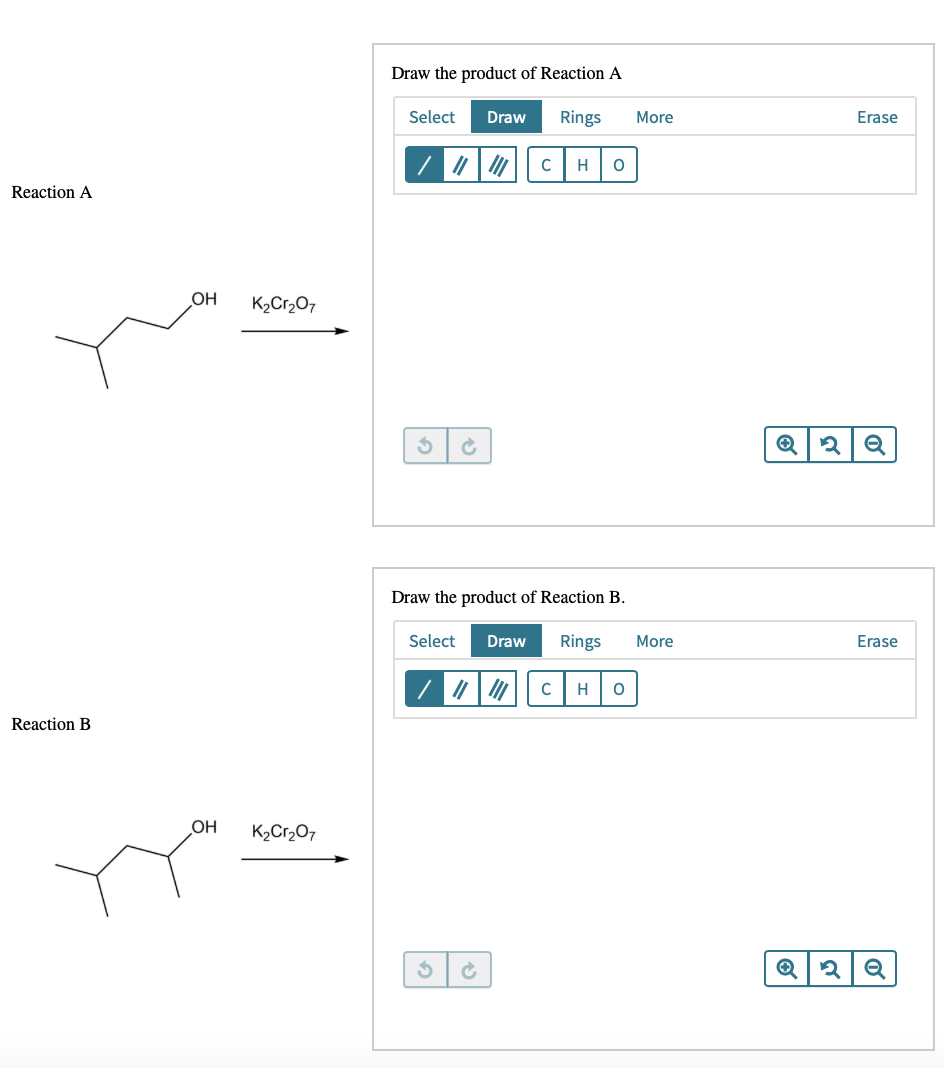
Transcribed Image Text:Draw the product of Reaction A
Select
Draw
Rings
More
Erase
H
Reaction A
OH
K2Cr,O7
Draw the product of Reaction B.
Select
Draw
Rings
More
Erase
| 7 || |
H
Reaction B
OH
K2Cr2O7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co