ducation.com/flow/connect.html (Thermochemistry: Energy FL.. i Saved 2 attempts left Check my work Be sure to answer all parts. Isooctane (C3H18; d=0.692 g/mL) is used as the fuel during a test of a new automobile engine. How much energy (in kJ) is released by complete combustion of 24.8 gal of isooctane to gases (AH =-5.44 x 10 kJ/mol)? Enter your answer in scientific notation. -3.09 < Prev 23 of 25 Next > arch 99+ DELL
ducation.com/flow/connect.html (Thermochemistry: Energy FL.. i Saved 2 attempts left Check my work Be sure to answer all parts. Isooctane (C3H18; d=0.692 g/mL) is used as the fuel during a test of a new automobile engine. How much energy (in kJ) is released by complete combustion of 24.8 gal of isooctane to gases (AH =-5.44 x 10 kJ/mol)? Enter your answer in scientific notation. -3.09 < Prev 23 of 25 Next > arch 99+ DELL
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.141QP
Related questions
Question
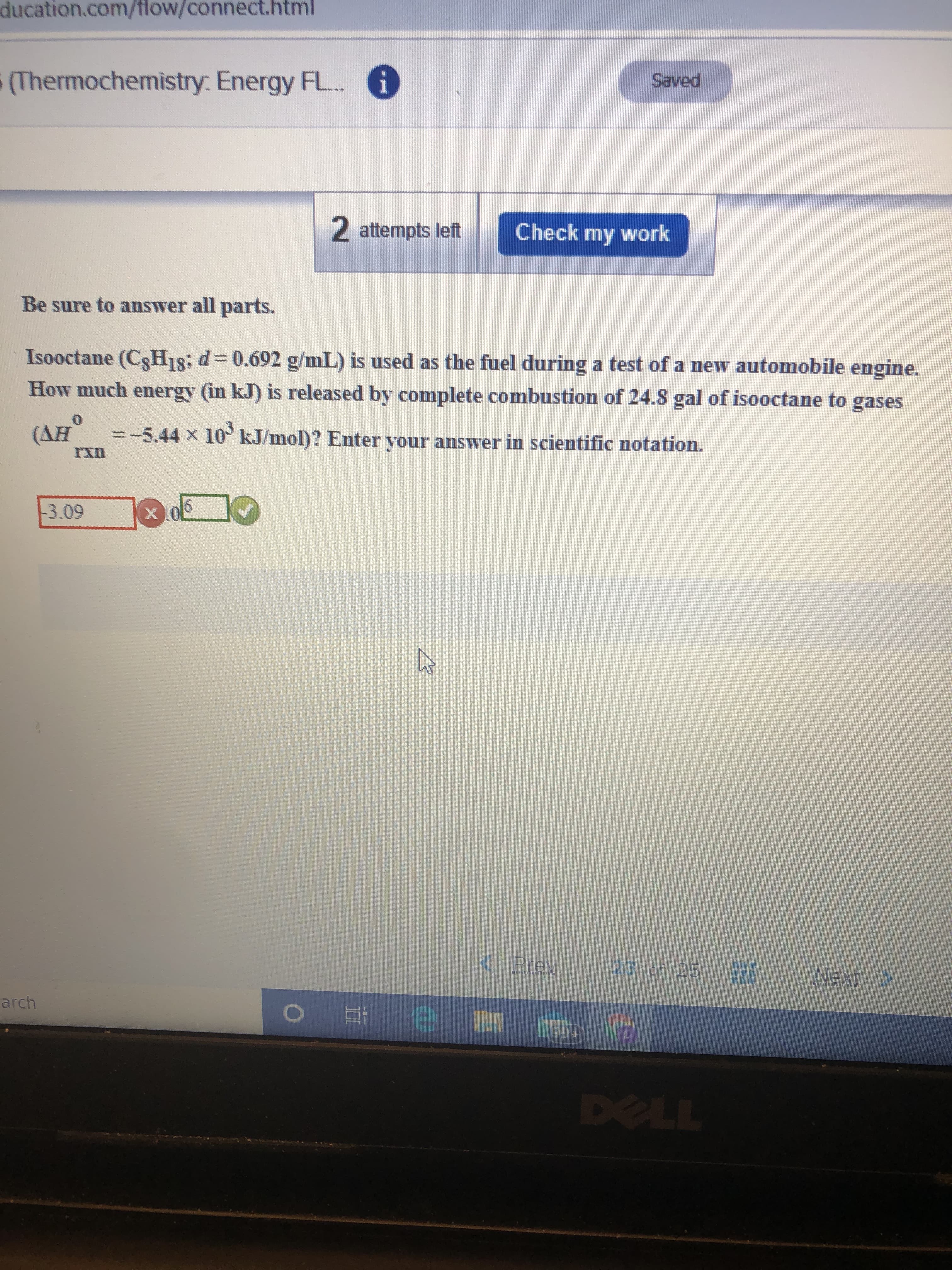
Transcribed Image Text:ducation.com/flow/connect.html
(Thermochemistry: Energy FL..
i
Saved
2 attempts left
Check my work
Be sure to answer all parts.
Isooctane (C3H18; d=0.692 g/mL) is used as the fuel during a test of a new automobile engine.
How much energy (in kJ) is released by complete combustion of 24.8 gal of isooctane to gases
(AH
=-5.44 x 10 kJ/mol)? Enter your answer in scientific notation.
-3.09
< Prev
23 of 25
Next >
arch
99+
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning