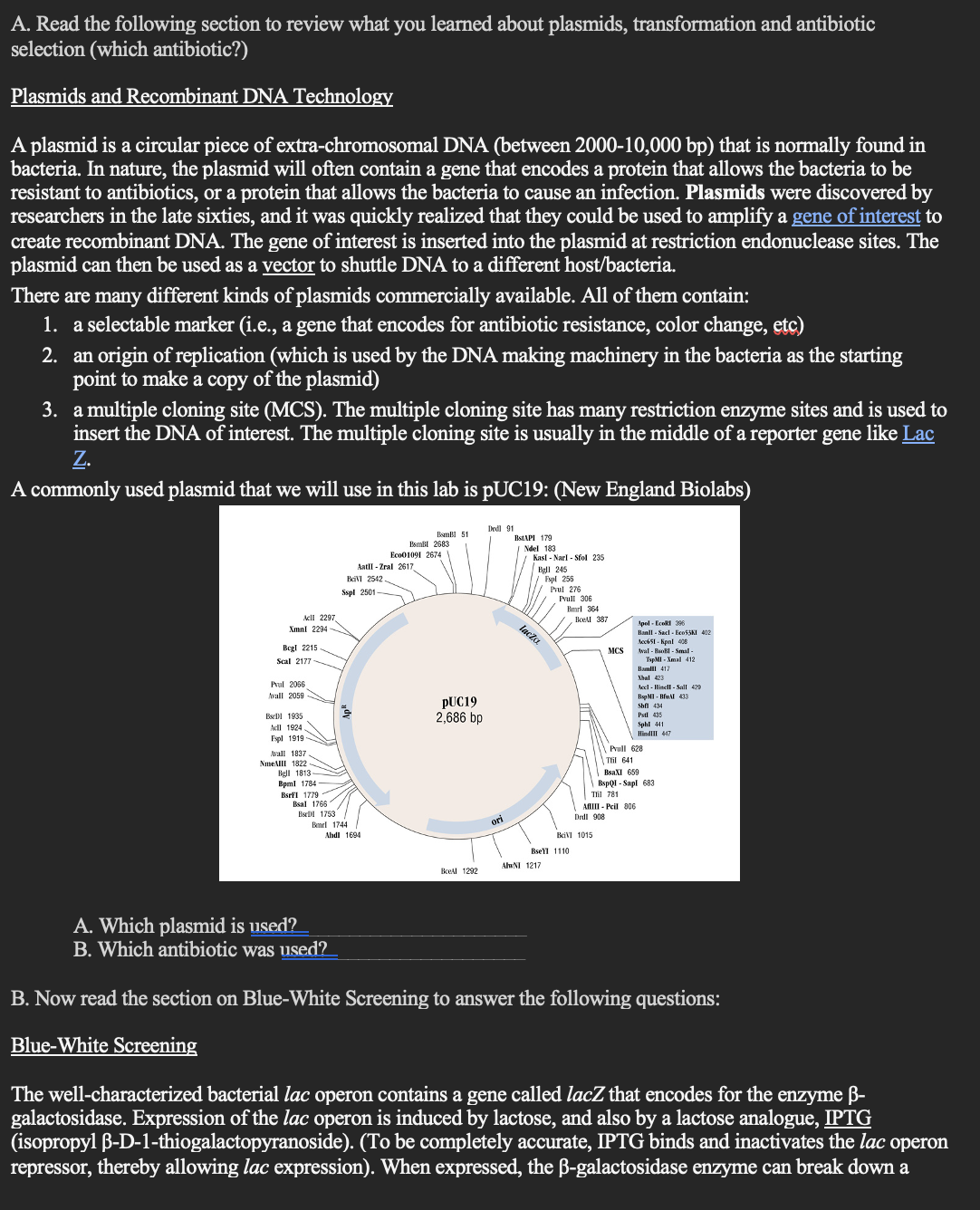
dye-linked substrate called X-gal (5-bromo-4-chloro-3-indolyl-ß-D-galacto-pyranoside) into galactose and an insoluble blue pigment (4-chloro-3-brom-indigo), which allows the bacterial colonies to appear BLUE on an agar plate. Most plasmid vectors, including pUC19, carry a short segment of lacZ that contains coding information for the first 146 amino acids of B-galactosidase. The host E. coli strains (ie, DH5a, which is used in this experiment) contain the lacZAM15 deletion mutation. When the plasmid vector is taken up by the bacteria, a functional ß- galactosidase enzyme is produced, which can break down X-gal to create blue colonies. If, however, the plasmid has a cloned gene of interested in the Multiple Cloning Site (pHPU-4), this will disrupt lacZ on the plasmid. If there is no lacZ on the plasmid to interact with the mutated lacZ in E. coli, no B- galactosisdase will be produced. Therefore, if X-gal is in the agar media, there is no enzyme to break it down so the colonies will appear white: What gene from the lac operon was cloned into the plasmid? What enzyme does that gene encode?. What is the lactose analog that we use to mimic lactose? What is the dye-linked suhstrate? What color colonies will be produced if E. coli can produce LacZ when grown with the analog and substrate? Color Fig. 1A below.
Bacterial Genomics
The study of the morphological, physiological, and evolutionary aspects of the bacterial genome is referred to as bacterial genomics. This subdisciplinary field aids in understanding how genes are assembled into genomes. Further, bacterial or microbial genomics has helped researchers in understanding the pathogenicity of bacteria and other microbes.
Transformation Experiment in Bacteria
In the discovery of genetic material, the experiment conducted by Frederick Griffith on Streptococcus pneumonia proved to be a stepping stone.
Plasmids and Vectors
The DNA molecule that exists in a circular shape and is smaller in size which is capable of its replication is called Plasmids. In other words, it is called extra-chromosomal plasmid DNA. Vectors are the molecule which is capable of carrying genetic material which can be transferred into another cell and further carry out replication and expression. Plasmids can act as vectors.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps









