Can i get help with these problems

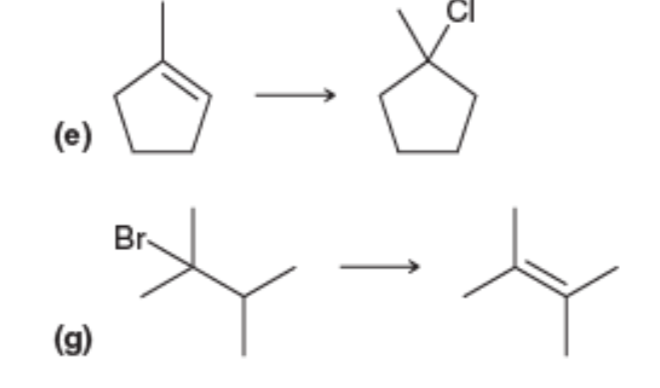
The addition reaction of an alkene with a hydrogen halide to furnish the haloalkane is termed as the hydrohalogenation reaction. In this reaction, the hybridized molecule is altered into the hybridized molecule at the point of hydrogen halide attack (Markovnikov's rule). The reaction proceeds via the formation of the carbocation intermediate. The higher the stability of the carbocation, the greater will the ease of its formation. The stability order of the carbocation: tertiary>secondary>primary (due to the hyperconjugation effects). The reaction of an alkyl halide with a strong base leads to the formation of an alkene. The reaction is termed as the elimination reaction. Based on the reaction conditions and the concentration of the nature of the alkyl halide, the pathway of the elimination reaction can be or .
e) HCl
The reaction of the alkene with HCl initially involves the electrophilic addition of the ion to the alkene to furnish the tertiary carbocation. Then the nucleophilic addition of the ion to the carbocation furnishes the addition product, a tertiary alkyl halide.
g)
When the secondary alkyl bromide is treated with a strong base , the base abstracts the products from the adjacent carbon atom in such a way that the more stable alkene is formed. According to Zaitsev's rule, the more substituted alkene is found to have greater stability than the less substituted alkene. Since the alkene product obtained is tetrasubstituted, it is the most stable alkene and hence the unsubstituted alkene product formation is neglected.
Step by step
Solved in 3 steps


