d. Photochemical reaction: For the above molecule a photon with n=2 (enough energy to promote one electron two energy levels up) hits it, reassign the HOMO energy level, project it on the above molecule and then form the cycle with proper stereochemistry.
d. Photochemical reaction: For the above molecule a photon with n=2 (enough energy to promote one electron two energy levels up) hits it, reassign the HOMO energy level, project it on the above molecule and then form the cycle with proper stereochemistry.
Chapter14: Conjugated Compounds And Ultraviolet Spectroscopy
Section14.SE: Something Extra
Problem 52AP
Related questions
Question
I only need part D!!! Thank you.
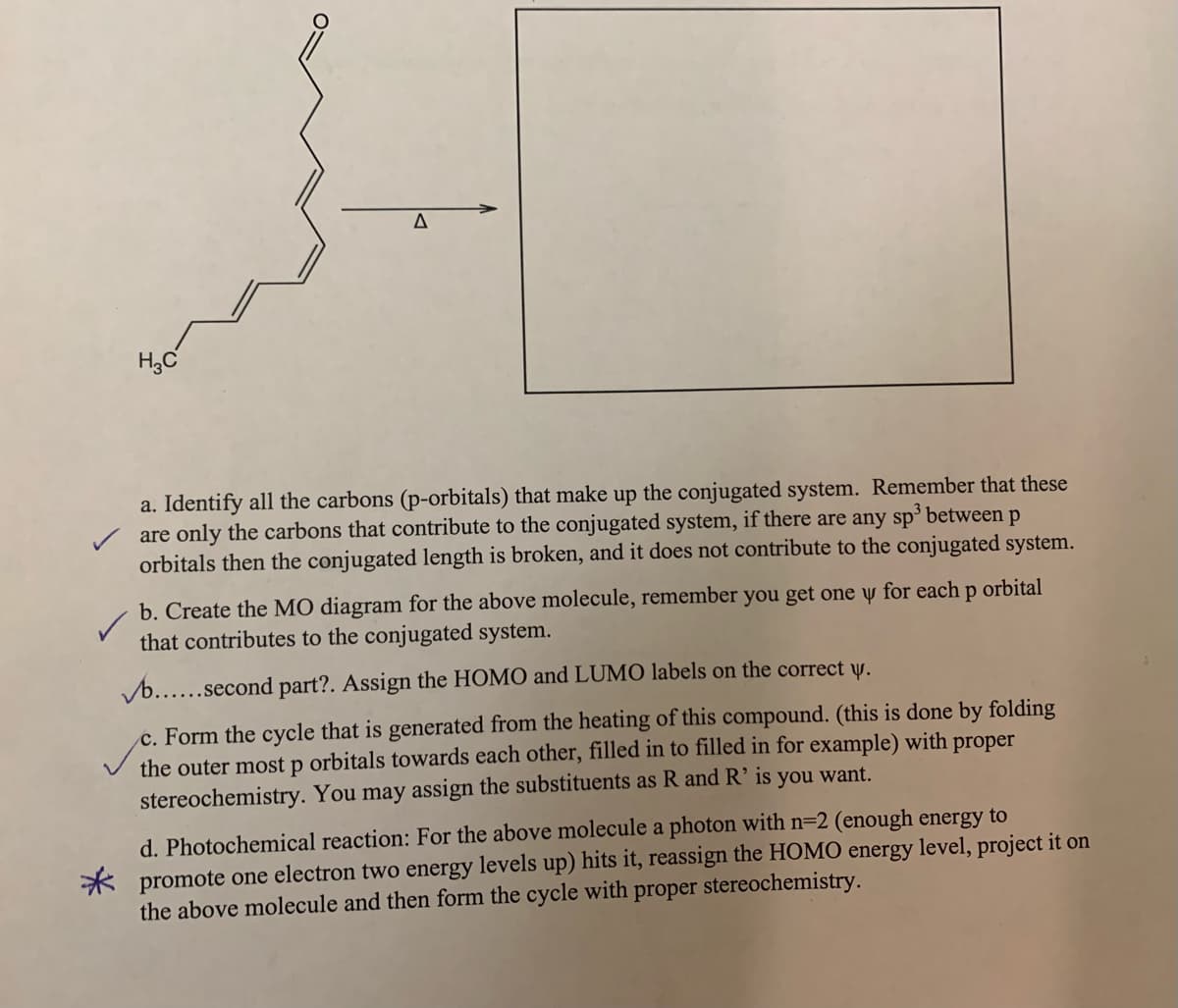
Transcribed Image Text:a. Identify all the carbons (p-orbitals) that make up the conjugated system. Remember that these
are only the carbons that contribute to the conjugated system, if there are any sp betweenp
orbitals then the conjugated length is broken, and it does not contribute to the conjugated system.
b. Create the MO diagram for the above molecule, remember you get one y for each p orbital
that contributes to the conjugated system.
Vb......second part?. Assign the HOMO and LUMO labels on the correct y.
c. Form the cycle that is generated from the heating of this compound. (this is done by folding
the outer most p orbitals towards each other, filled in to filled in for example) with proper
stereochemistry. You may assign the substituents as R and R’is you want.
d. Photochemical reaction: For the above molecule a photon with n=2 (enough energy to
* promote one electron two energy levels up) hits it, reassign the HOMO energy level, project it on
the above molecule and then form the cycle with proper stereochemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning