Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.40P: Enamines normally react with methyl iodide to give two products: one arising from alkylation at...
Related questions
Question
a) Give the mechanistic symbols (SN1, SN2, E1, E2) that are most consistent with each of the following statements:
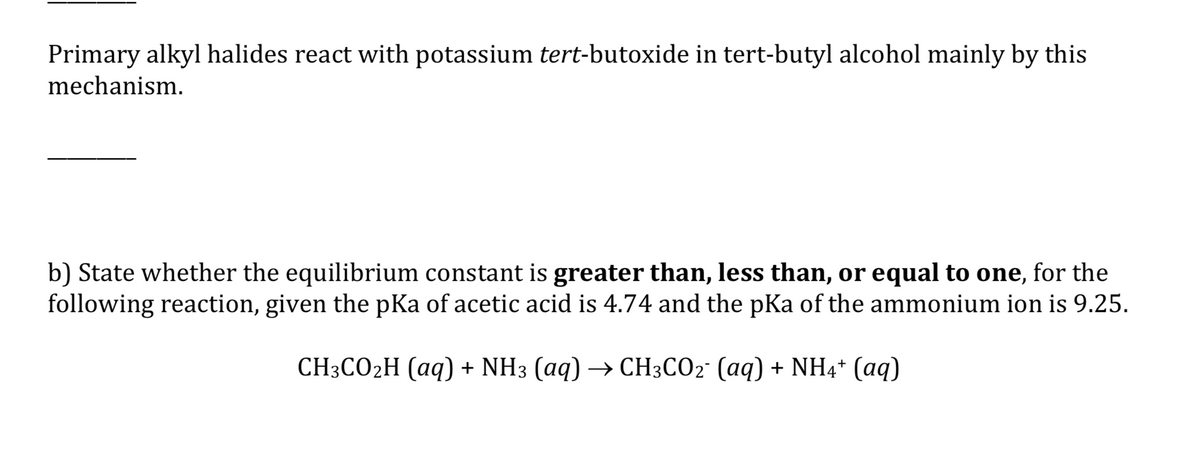
Transcribed Image Text:Primary alkyl halides react with potassium tert-butoxide in tert-butyl alcohol mainly by this
mechanism.
b) State whether the equilibrium constant is greater than, less than, or equal to one, for the
following reaction, given the pKa of acetic acid is 4.74 and the pKa of the ammonium ion is 9.25.
СH:СО2H (аq) + NH3 (аq) —> СH3CO2: (аq) + NH4* (аq)

Transcribed Image Text:Unhindered primary alkyl halides react with sodium ethoxide in ethanol mainly by this
mechanism.
When cyclohexyl bromide is treated with sodium tert-butoxide in tert-butanol, the major
product is formed by this mechanism.
The substitution product obtained by solvolysis of tert-butyl bromide in ethanol arises by this
mechanism.
In ethanol that contains a dilute amount of sodium ethoxide, tert-butyl bromide reacts mainly by
this mechanism.
When 2-bromobutane is treated with potassium cyanide in DMSO, the major product is formed
by this mechanism.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
