E. 50 mL beaker 20.127 9 Mass of beaker Volume of water measured in each trial 10.00ML Calculated density of water (g/mL) Mass of beaker Trial Mass of water (g) and water (g) 31.532g 37.236 g 37.6259 31.3279 1 3 Average density Percent error in density Standard deviation in density 4)
E. 50 mL beaker 20.127 9 Mass of beaker Volume of water measured in each trial 10.00ML Calculated density of water (g/mL) Mass of beaker Trial Mass of water (g) and water (g) 31.532g 37.236 g 37.6259 31.3279 1 3 Average density Percent error in density Standard deviation in density 4)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 66SCQ
Related questions
Question
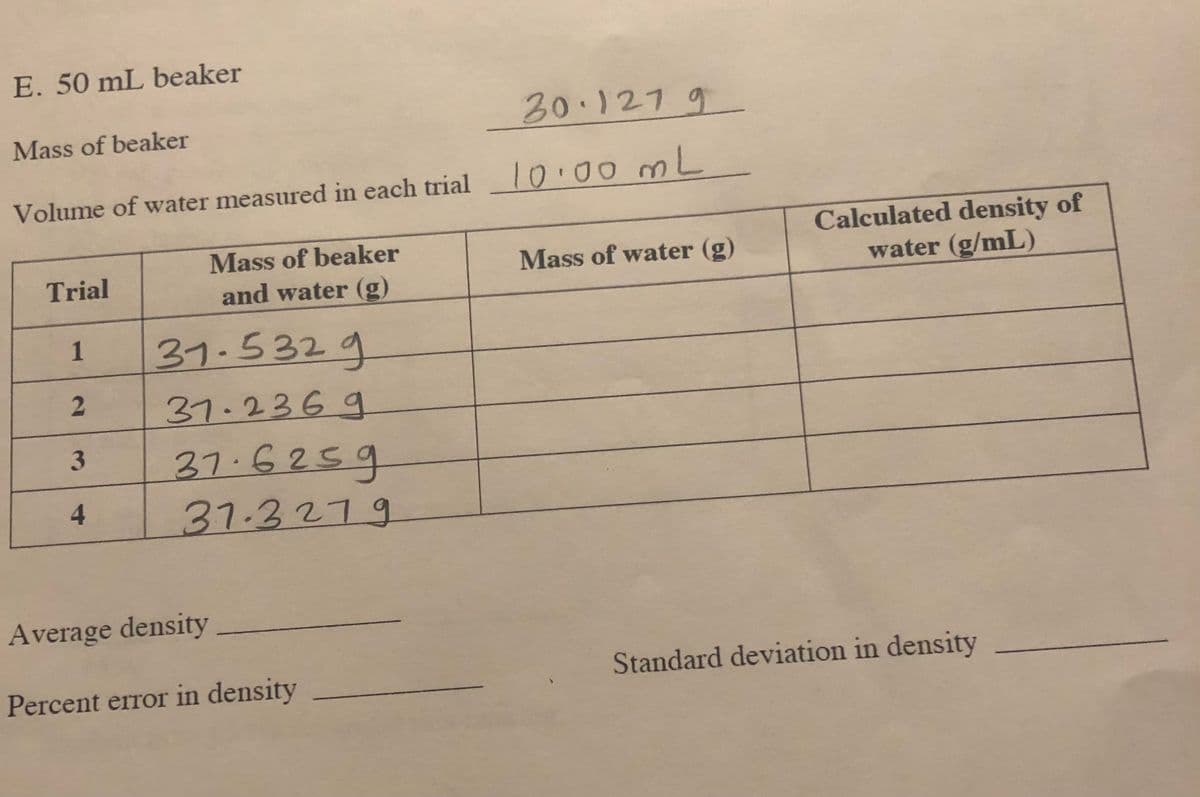
Transcribed Image Text:E. 50 mL beaker
20.1279
Mass of beaker
10.00ML
Volume of water measured in each trial
Calculated density of
water (g/mL)
Mass of beaker
Trial
Mass of water (g)
and water (g)
1
31.5329
37.236g
37.6259
4
31.3279
Average density
Percent error in density
Standard deviation in density
2.

Transcribed Image Text:10. Create a hypothesis for this experiment- do this without looking at the internet or other
reference sources. You will not be marked incorrect for your choices as long as your
explanation is logical.
Which compound will have a higher viscosity?
Ethanol
Acetone
Which compound will have a higher surface tension?
Ethanol
Acetone
Which compound will have a higher volatility?
Ethanol
Acetone
Which compound will have a stronger capillary action?
Ethanol
Acetone
Which compound will have a higher boiling point?
Ethanol
Acetone
Which compound(s) will be soluble in water?
Ethanol
Acetone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
