E. Calculate AH for the reaction CH4 e + NH3 () → HCN (2) + 3 H2 (=); given: N2 e+3 H2 e → 2 NH3 e C) + 2 H2 (2) → CH4 (2) H2 e+ 2 C (3) + N2 (2) → 2 HCN E AH = -91.8 kJ AH = -74.9 kJ AH = +270.3 kJ
E. Calculate AH for the reaction CH4 e + NH3 () → HCN (2) + 3 H2 (=); given: N2 e+3 H2 e → 2 NH3 e C) + 2 H2 (2) → CH4 (2) H2 e+ 2 C (3) + N2 (2) → 2 HCN E AH = -91.8 kJ AH = -74.9 kJ AH = +270.3 kJ
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.8: Measuring Reaction Enthalpies: Calorimetry
Problem 4.9PSP
Related questions
Question
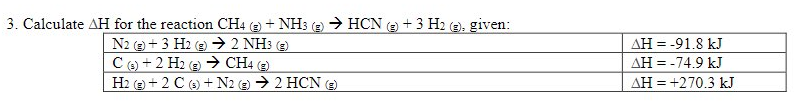
Transcribed Image Text:3. Calculate AH for the reaction CH4 (e + NH3 (e) > HCN ( + 3 H2 (2), given:
N2 + 3 H2 (9 → 2 NH3 ()
AH = -91.8 kJ
Ce) + 2 H2 e → CH (e
e)
AH = -74.9 kJ
H2 e + 2 C (6) +N2 e) → 2 HCN e)
AH = +270.3 kJ

Transcribed Image Text:Task 3.2. Calculate the standard enthalpy of reaction of some thermochemical equations governed by Hess Law.
Direction: Solve the following items. Show complete solution. Express all standard enthalpy to kilojoules.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning