Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.75QE
Related questions
Question
35. just give me the answer asap thanks
![1
2
H
Не
1.008
4.00
3
5
6.
10
Ве
В
Ne
6.94
9.01
10.81
12.01
14.00
16.00
19.00
20.18
11
12
13
14
15
16
17
18
Na Mg
Al
Ar
22.99
24.31
26.98
28.09
30.97
32.06
35.45
39.95
19
20
21
22
23
24
25
26
27
28
29
30
31
32
34
35
36
Mn
Ga | Ge
Kr
39.10
40.10
44.96
47.90
50.94
52.00
54.94
55.85
58.93
58.70
63.55
65.38
69.72
72.59
74.92
78.96
79.90
83.80
37
38
39
41
42
45
46
49
52
53
54
Rb
Nb Mo
Pd Ag
Хе
85.47
87.62
88.91
91.22
92.91.
95.94
[97.91) 101.07
102.91
106.4
107.87
112.41
114.82
118.69
121.75
127.60
126.90
131.30
55
56
57-71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Cs
Та
Rn
132.91
137.33
178.49
180.95
183.85
186.21
190.2
192.22
195.05
196.97
200.59
204.37
207.2
208.98
[209]
[210]
[222]
87
88
89-103
104
105
106
107
108
109
110
112
113
114
115
116
117
118
Fr
Ra
1223.02) (226.03]
Db Sg
Rg | Cn Nh
Og
[294]
Mt
FI
[265.12 1268.131 [271.131 (270) 1277.15|| [276.15) (281.16)] 280.16] (285.17) (284.18]|1289.19)|1288.191 [293]
[294]
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Lanthanides La
Pr
Nd Pm Sm Eu
173.04 174.97
138.91
89
140.12
140.91
144.24
[145]
150.4
151.96
157.25
158.93
162.50
164.93
167.26
168.93
91
92
93
94
95
96
97
99
100
101
102
Pa
231.04 238.029 [237.05] [244.06] [243.06] [247.07] [247.07] [251.08] [252.08] [257.10] [258.10] [259.10] [262.11]
Np Pu Am Cm
Fm M
Actinides
[277.03] 232.04](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F820fec86-f385-4ae8-a90f-7ecf5832d665%2Fc5fc9b5e-ad33-4234-8892-5783a063ff33%2Fpb57dbh_processed.jpeg&w=3840&q=75)
Transcribed Image Text:1
2
H
Не
1.008
4.00
3
5
6.
10
Ве
В
Ne
6.94
9.01
10.81
12.01
14.00
16.00
19.00
20.18
11
12
13
14
15
16
17
18
Na Mg
Al
Ar
22.99
24.31
26.98
28.09
30.97
32.06
35.45
39.95
19
20
21
22
23
24
25
26
27
28
29
30
31
32
34
35
36
Mn
Ga | Ge
Kr
39.10
40.10
44.96
47.90
50.94
52.00
54.94
55.85
58.93
58.70
63.55
65.38
69.72
72.59
74.92
78.96
79.90
83.80
37
38
39
41
42
45
46
49
52
53
54
Rb
Nb Mo
Pd Ag
Хе
85.47
87.62
88.91
91.22
92.91.
95.94
[97.91) 101.07
102.91
106.4
107.87
112.41
114.82
118.69
121.75
127.60
126.90
131.30
55
56
57-71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Cs
Та
Rn
132.91
137.33
178.49
180.95
183.85
186.21
190.2
192.22
195.05
196.97
200.59
204.37
207.2
208.98
[209]
[210]
[222]
87
88
89-103
104
105
106
107
108
109
110
112
113
114
115
116
117
118
Fr
Ra
1223.02) (226.03]
Db Sg
Rg | Cn Nh
Og
[294]
Mt
FI
[265.12 1268.131 [271.131 (270) 1277.15|| [276.15) (281.16)] 280.16] (285.17) (284.18]|1289.19)|1288.191 [293]
[294]
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Lanthanides La
Pr
Nd Pm Sm Eu
173.04 174.97
138.91
89
140.12
140.91
144.24
[145]
150.4
151.96
157.25
158.93
162.50
164.93
167.26
168.93
91
92
93
94
95
96
97
99
100
101
102
Pa
231.04 238.029 [237.05] [244.06] [243.06] [247.07] [247.07] [251.08] [252.08] [257.10] [258.10] [259.10] [262.11]
Np Pu Am Cm
Fm M
Actinides
[277.03] 232.04
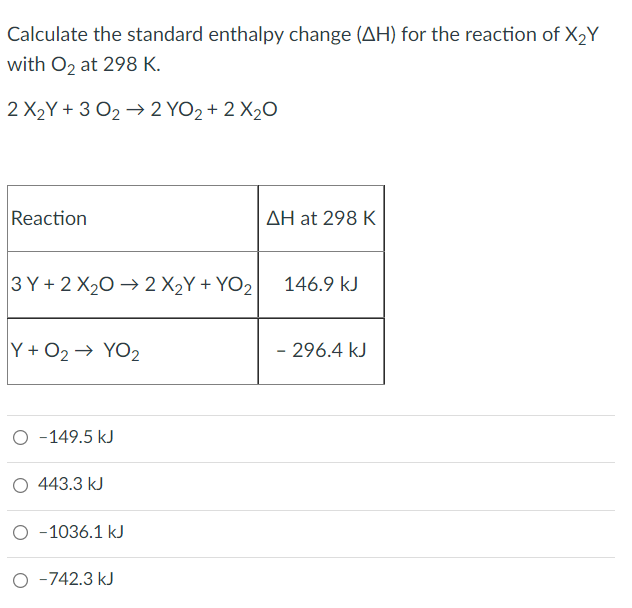
Transcribed Image Text:Calculate the standard enthalpy change (AH) for the reaction of X2Y
with O, at 298 K.
2 X2Y + 3 O2 → 2 YO2 + 2 X20
Reaction
AH at 298 K
3 Y + 2 X20 → 2 X2Y + YO2
146.9 kJ
Y + O2→ YO2
- 296.4 kJ
O -149.5 kJ
O 443.3 kJ
-1036.1 kJ
-742.3 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning