e. Notice that the face atom and the corner atoms along the diagonal all touch. Solve for the length of the diagonal in terms of "r" the radius of one atom. Answer: f. Since question 3d and 3e express the length of the diagonal when the side is "a" set them equal and solve for "a", the side of length of a face- centered unit cube. Answer: g. Find the total volume of the atoms in the face-centered unit cell expressed in terms of r. Use commor equations to find the volume of atoms in the unit cell. To do this, take the volume of a sphere in terms of "r" and multiply it by the total number of atoms represented in the unit cell. Answer:
e. Notice that the face atom and the corner atoms along the diagonal all touch. Solve for the length of the diagonal in terms of "r" the radius of one atom. Answer: f. Since question 3d and 3e express the length of the diagonal when the side is "a" set them equal and solve for "a", the side of length of a face- centered unit cube. Answer: g. Find the total volume of the atoms in the face-centered unit cell expressed in terms of r. Use commor equations to find the volume of atoms in the unit cell. To do this, take the volume of a sphere in terms of "r" and multiply it by the total number of atoms represented in the unit cell. Answer:
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.6QAP
Related questions
Question
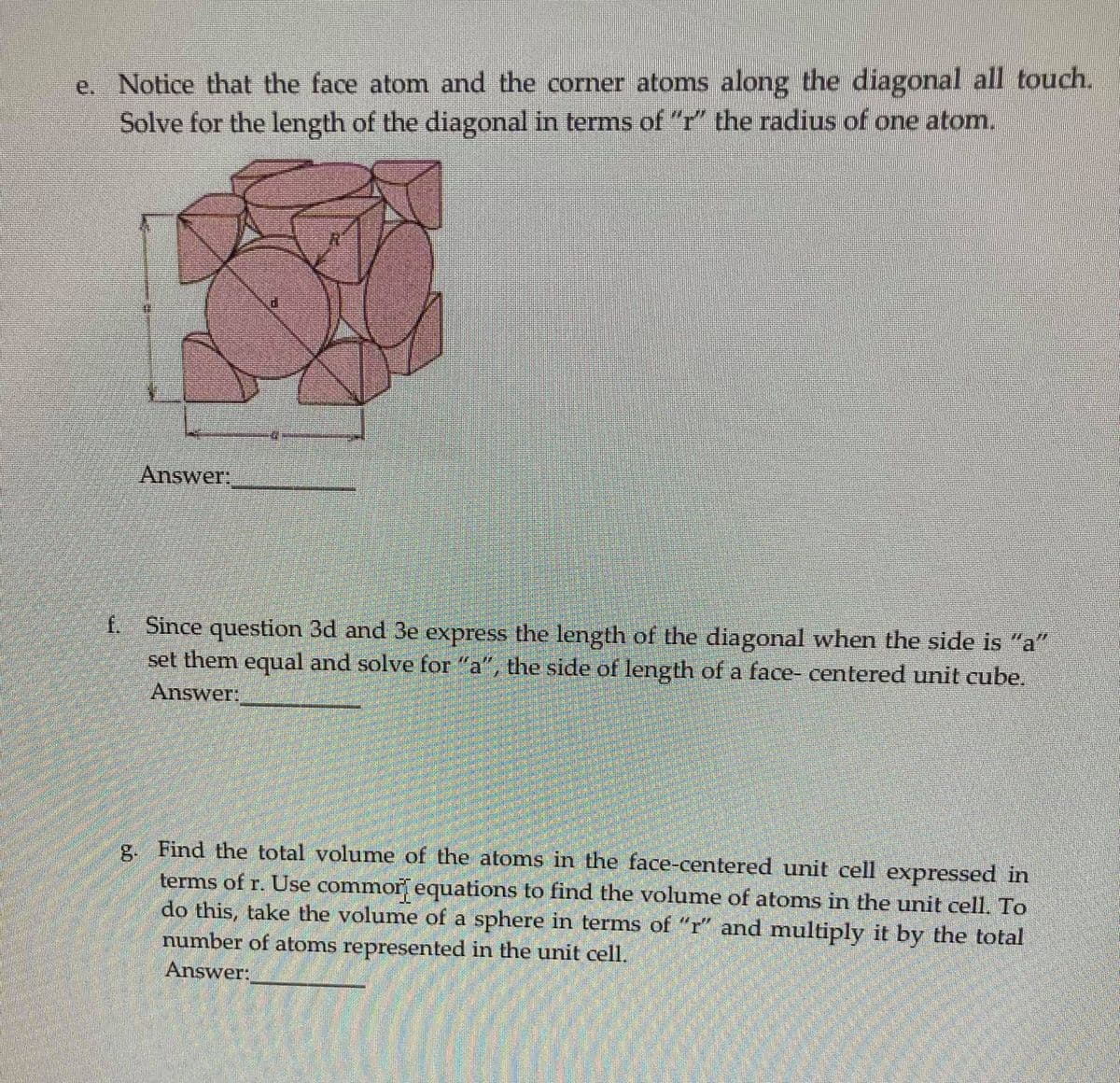
Transcribed Image Text:e. Notice that the face atom and the corner atoms along the diagonal all touch.
Solve for the length of the diagonal in terms of "r" the radius of one atom.
Answer:
f. Since question 3d and 3e express the length of the diagonal when the side is "a"
set them equal and solve for "a", the side of length of a face- centered unit cube.
Answer:
g. Find the total volume of the atoms in the face-centered unit cell expressed in
terms of r. Use commor equations to find the volume of atoms in the unit cell. To
do this, take the volume of a sphere in terms of "r" and multiply it by the total
number of atoms represented in the unit cell.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

