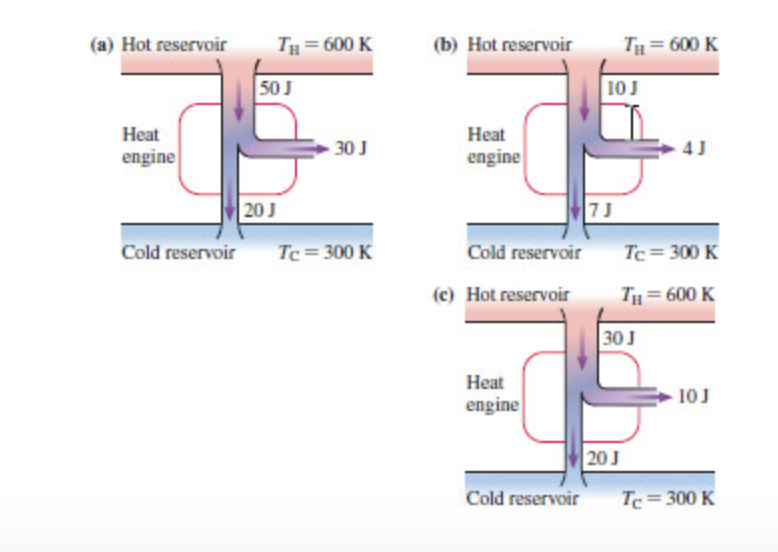
ease help me! Thank you so much!!! A.) For each engine calculate ΔE = QH−Wout−QC, where QH is the amount of heat transferred from the hot reservoir, QC is the amount of heat transferred to the cold reservoir, Wout is the energy output of the heat engine. QH, Wout, QC are positive quantities. B.) Which, if any, of the heat engines violate(s) the first law of thermodynamics? C.) For each engine calculate the theoretical maximum efficiency (Carnot efficiency) emax. D.) For each engine calculate the actual efficiency e.
Hi there! I am in desperate need of help. I cannot figure this out. Here is my diagram of the heat engines. Please help me! Thank you so much!!!
A.) For each engine calculate ΔE = QH−Wout−QC, where QH is the amount of
B.) Which, if any, of the heat engines violate(s) the first law of
C.) For each engine calculate the theoretical maximum efficiency (Carnot efficiency) emax.
D.) For each engine calculate the actual efficiency e.
E.) Which, if any, of the heat engines violate(s) the second law of thermodynamics?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









