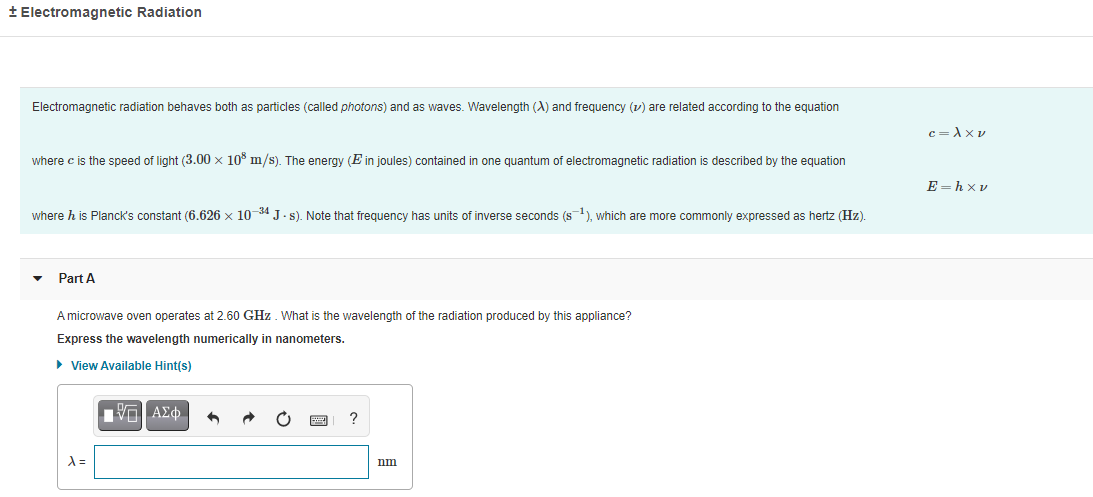
Electromagnetic radiation behaves both as particles (called photons) and as waves. Wavelength (A) and frequency (v) are related according to the equation c =A x v where c is the speed of light (3.00 × 10% m/s). The energy (E in joules) contained in one quantum of electromagnetic radiation is described by the equation E -hx v where h is Planck's constant (6.626 x 10 34 J. s). Note that frequency has units of inverse seconds (s1), which are more commonly expressed as hertz (Hz). Part A A microwave oven operates at 2.60 GHz. What is the wavelength of the radiation produced by this appliance? Express the wavelength numerically in nanometers.
Electromagnetic radiation behaves both as particles (called photons) and as waves. Wavelength (A) and frequency (v) are related according to the equation c =A x v where c is the speed of light (3.00 × 10% m/s). The energy (E in joules) contained in one quantum of electromagnetic radiation is described by the equation E -hx v where h is Planck's constant (6.626 x 10 34 J. s). Note that frequency has units of inverse seconds (s1), which are more commonly expressed as hertz (Hz). Part A A microwave oven operates at 2.60 GHz. What is the wavelength of the radiation produced by this appliance? Express the wavelength numerically in nanometers.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 120QRT
Related questions
Question
Transcribed Image Text:t Electromagnetic Radiation
Electromagnetic radiation behaves both as particles (called photons) and as waves. Wavelength (A) and frequency (v) are related according to the equation
c= Axv
where c is the speed of light (3.00 x 10% m/s). The energy (E in joules) contained in one quantum of electromagnetic radiation is described by the equation
E =hxv
where h is Planck's constant (6.626 x 10 34 J. s). Note that frequency has units of inverse seconds (s), which are more commonly expressed as hertz (Hz).
Part A
A microwave oven operates at 2.60 GHz. What is the wavelength of the radiation produced by this appliance?
Express the wavelength numerically in nanometers.
• View Available Hint(s)
nV ΑΣφ.
?
nm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning