ell. Begin with 2.45 mol Cu and multiply by Avoga- 6.022 X 1U SI P Nn = 1.48 X 10 Cu atoms mass tha 24 2.45 mol Cu X dro's number to get to the number of Cu atoms. 1 mol Cu The CHECK Since atoms are small, it makes sense that the answer is large. The given number of moles of copper is almost 2.5, so the number of atoms is almost 2.5 times Avogadro's number. of that FOR PRACTICE 2.6 A pure silver ring contains 2.80 x 1022 silver atoms. How many moles of silver atoms does it contain? Exam 22 allezo9
ell. Begin with 2.45 mol Cu and multiply by Avoga- 6.022 X 1U SI P Nn = 1.48 X 10 Cu atoms mass tha 24 2.45 mol Cu X dro's number to get to the number of Cu atoms. 1 mol Cu The CHECK Since atoms are small, it makes sense that the answer is large. The given number of moles of copper is almost 2.5, so the number of atoms is almost 2.5 times Avogadro's number. of that FOR PRACTICE 2.6 A pure silver ring contains 2.80 x 1022 silver atoms. How many moles of silver atoms does it contain? Exam 22 allezo9
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 120AP
Related questions
Question
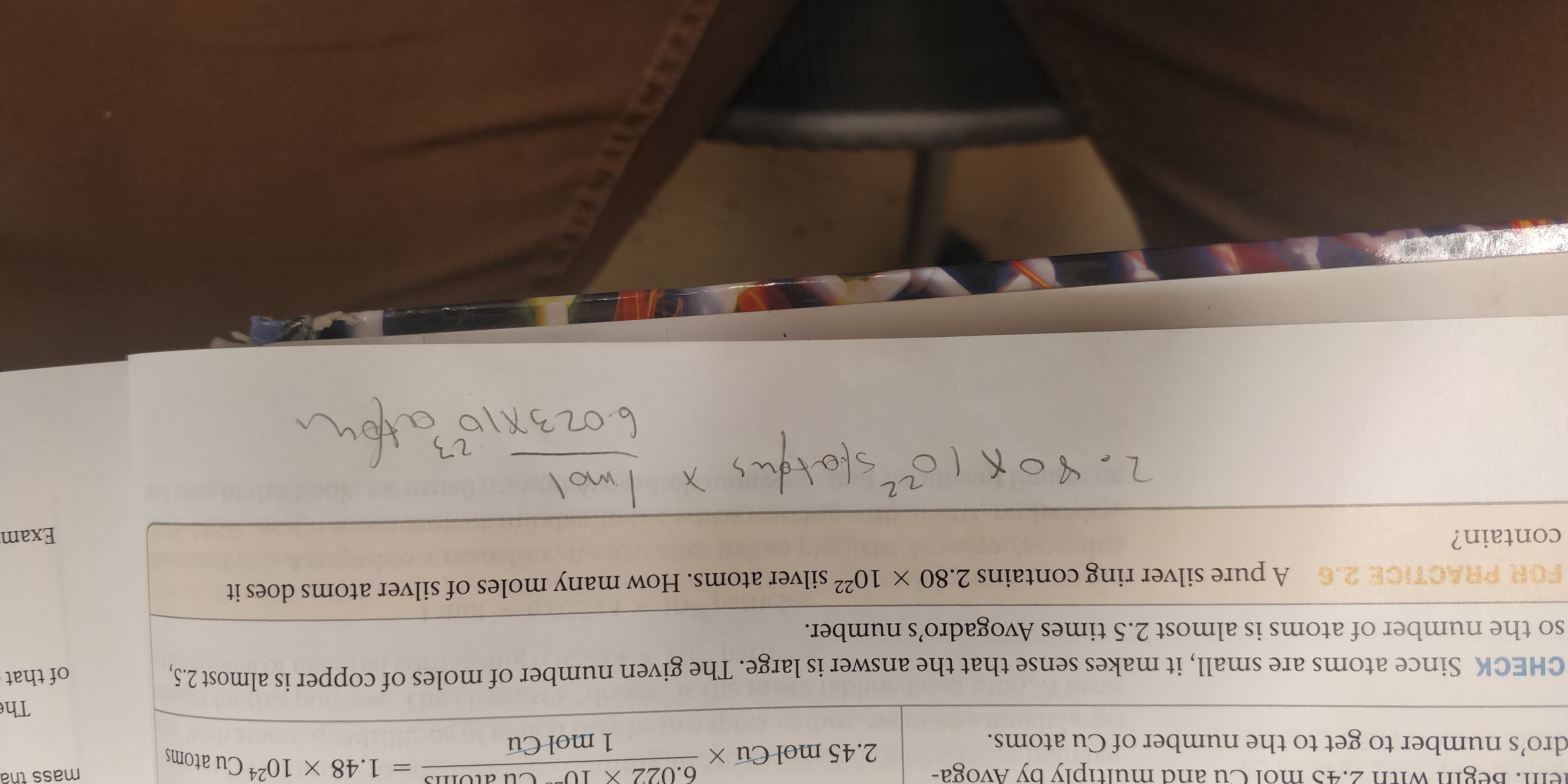
Transcribed Image Text:ell. Begin with 2.45 mol Cu and multiply by Avoga-
6.022 X 1U
SI P Nn
= 1.48 X 10 Cu atoms
mass tha
24
2.45 mol Cu X
dro's number to get to the number of Cu atoms.
1 mol Cu
The
CHECK Since atoms are small, it makes sense that the answer is large. The given number of moles of copper is almost 2.5,
so the number of atoms is almost 2.5 times Avogadro's number.
of that
FOR PRACTICE 2.6 A pure silver ring contains 2.80 x 1022 silver atoms. How many moles of silver atoms does it
contain?
Exam
22
allezo9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning