Empirical Formula of a Hydrate Calculation Sheet (Data Table Calculations): (Show how you (h) calculated items (d) though (1) - of the Data Table. Please write clearly so that your instructor (i) can determine how you calculated your answers. (1) molar mass of H2O (d) (k) moles of H20 in unknown hydrate (e) (1) ratio of moles water in unknown hydrate to moles of ZNSO4 (g)
Empirical Formula of a Hydrate Calculation Sheet (Data Table Calculations): (Show how you (h) calculated items (d) though (1) - of the Data Table. Please write clearly so that your instructor (i) can determine how you calculated your answers. (1) molar mass of H2O (d) (k) moles of H20 in unknown hydrate (e) (1) ratio of moles water in unknown hydrate to moles of ZNSO4 (g)
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 1P
Related questions
Question

Transcribed Image Text:Empirical Formula of a Hydrate
Calculation Sheet (Data Table
Calculations):_(Show how you
calculated items (d) though (1)
(h)
of the Data Table. Please write
clearly so that your instructor
determine
(i)
can
how
calculated your answers.
you
(1) molar mass of H2O
(d)
(k) moles of H20 in unknown hydrate
(e)
(1) ratio of moles water in unknown
hydrate to moles of ZnSO4
(f)
(g)
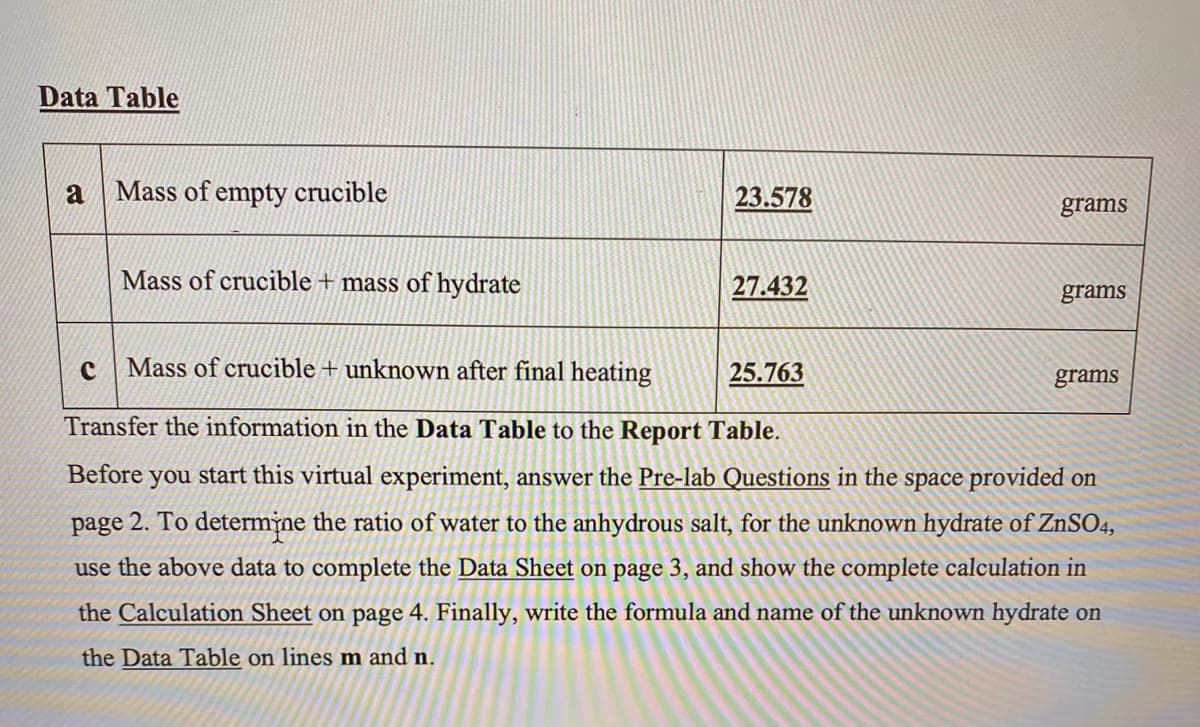
Transcribed Image Text:Data Table
Mass of empty crucible
23.578
grams
Mass of crucible + mass of hydrate
27.432
grams
Mass of crucible + unknown after final heating
25.763
grams
Transfer the information in the Data Table to the Report Table.
Before
you start this virtual experiment, answer the Pre-lab Questions in the space provided on
page 2. To determine the ratio of water to the anhydrous salt, for the unknown hydrate of ZnSO4,
use the above data to complete the Data Sheet on page 3, and show the complete calculation in
the Calculation Sheet on page 4. Finally, write the formula and name of the unknown hydrate on
the Data Table on lines m and n.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

